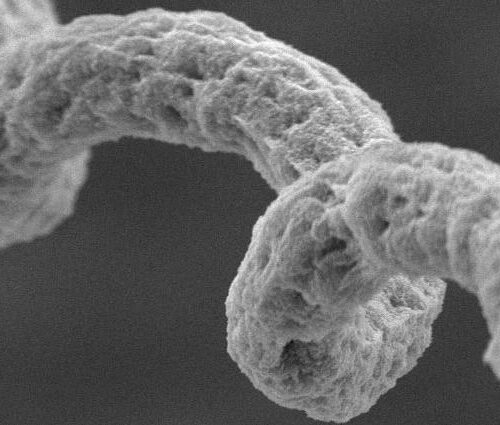
Peer-Reviewed Publication Researchers at the University of Science and Technology of China have developed a soft robotic “finger” with a sophisticated sense of touch that can perform routine doctor office examinations, including taking a patient’s pulse and checking for abnormal lumps. This work was published October 9 in the Cell Press journal Cell Reports Physical Science....
Category: <span>Devices</span>
Ablation Technology: Innovations and Industry Overview
What is ablation technology? Ablation therapy consists of a minimally invasive treatment, which can be used for treating cancer by destroying tumors and other abnormal tissues within the body. It uses either extremely high or low temperatures to remove a layer or layers of tissue. However, radiofrequency energy or electrical currents can also be used.1...
Revolutionizing cardiovascular risk assessment with AI
University of Pennsylvania School of Engineering and Applied Science A recent position paper in the Asia-Pacific Journal of Ophthalmology explores the transformative potential of artificial intelligence (AI) in ophthalmology. Led by Lama Al-Aswad, Professor of Ophthalmology and Irene Heinz Given and John La Porte Given Research Professor of Ophthalmology II, of the Scheie Eye Institute, the work represents a...
Magnetic Tentacle Robots for Minimally Invasive Procedures
Researchers at the University of Leeds in the UK have developed a magnetic tentacle robot that is intended for use in minimally invasive medical procedures, such as the treatment of tumors in the lungs. The soft tentacles are made from silicone. They are unlikely to cause tissue damage, and contain a series of magnets that...
Best Buy Health shows first signs of success with Geek Squad helping at-home patients
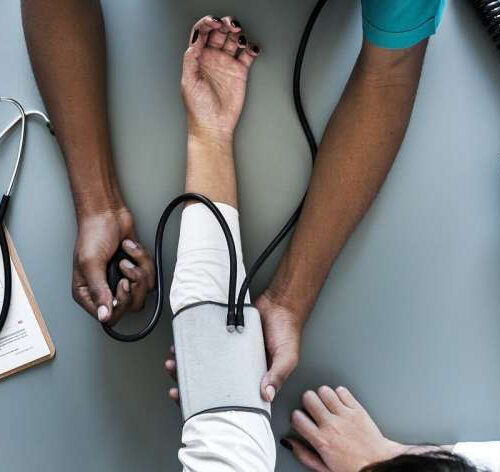
by Nicole Norfleet, Star Tribune blood pressure Credit: CC0 Public DomainThat next visit from one of Best Buy’s Geek Squad workers might not be for help with a television or to install a speaker but instead to learn about a blood pressure cuff or set up a pulse oximeter, which then sends readings straight to...
Mask Type Affects Airway Collapsibility in OSA
Heidi Splete April 07, 2023 Use of an oronasal mask for delivery of continuous positive airway pressure (CPAP) was associated with increased upper airway collapsibility and the need for greater therapeutic pressure compared to nasal masks, as indicated by data from 14 individuals with obstructive sleep apnea (OSA). Previous research has linked oronasal mask use to lower adherence, higher residual apnea...
Three Wild Health Technologies in (or Close to) Clinical Trials
David Warmflash, MD February 03, 2023 When I was a child, I watched syndicated episodes of the original Star Trek. I was dazzled by the space travel, sure, but also the medical technology. A handheld “tricorder” detected diseases, while an intramuscular injector (“hypospray”) could treat them. Sickbay “biobeds” came with real-time health monitors that looked futuristic at...
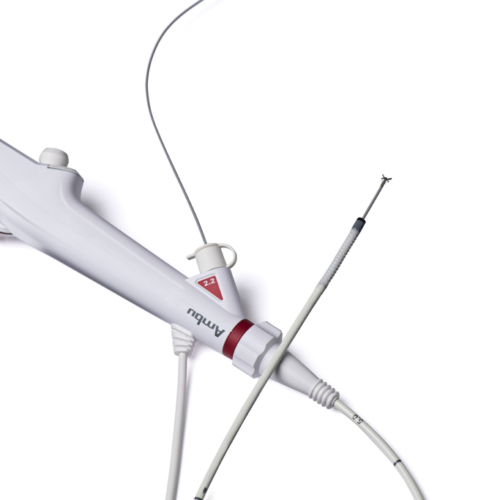
AMBU ANNOUNCES FDA CLEARANCE OF FIFTH-GENERATION SINGLE-USE BRONCHOSCOPE
JULY 29TH, 2022 AMBU RELEASES, RELEASES – FEATURED Ambu will extend market leadership in pulmonology by entering the bronchoscopy suite segment following U.S. and European market clearances. COLUMBIA, MD – July 29, 2022 – Ambu Inc. announces that Ambu® aScopeTM 5 Broncho, a family of single-use, sterile bronchoscopes, has received 510(k) regulatory clearance from the U.S. Food...
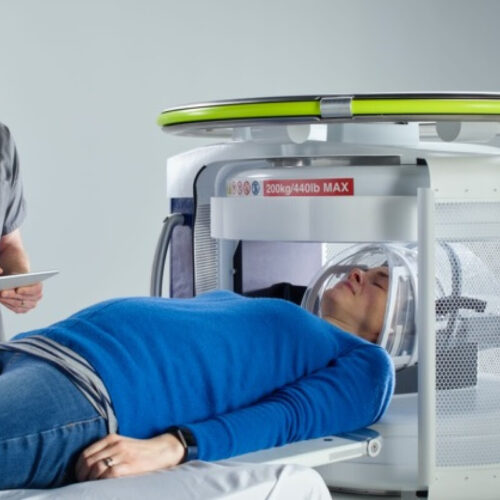
Portable MRI can accurately detect most common form of stroke
By Rich Haridy April 20, 2022 A portable MRI machine effectively detected ischemic stroke in 45 out of 50 patients Hyperfine New research has found the world’s first portable MRI machine can accurately detect ischemic strokes. The machine was cleared for use by the US Food & Drug Administration (FDA) in 2020 and researchers are...
FDA warns of cyber vulnerabilities in medical device software components
Greg Slabodkin Senior Editor JuSun via Getty Images Dive Brief: FDA on Tuesday issued a cybersecurity alert warning about seven vulnerabilities in software company PTC’s Axeda agent and desktop server, third-party components that enable remote service via the internet, which are used in medical devices from several manufacturers. The agency said if the vulnerabilities in the web-based agent and desktop...