Megan Brooks The US Food and Drug Administration (FDA) has approved golodirsen injection (Vyondys 53, Sarepta Therapeutics), the first treatment for Duchenne muscular dystrophy (DMD) in patients with a confirmed mutation amenable to exon 53 skipping. Last summer, the agency declined to approve of the drug on an accelerated basis, sending a complete response letter...
Tag: <span>Duchenne Muscular Dystrophy</span>
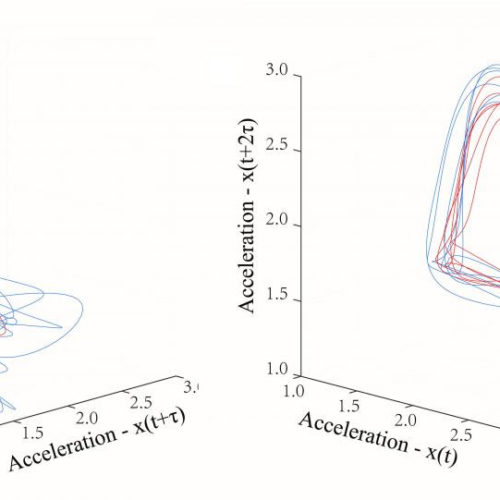
Duchenne muscular dystrophy diagnosis improved by simple accelerometers
As the most common form of the disease, early diagnosis of Duchenne muscular dystrophy is key to survival AMERICAN INSTITUTE OF PHYSICS WASHINGTON, February 11, 2020 — Duchenne muscular dystrophy is the most common type of muscular dystrophy, affecting more than 10,000 males at birth per year in the United States with severe physical disability,...
Spironolactone noninferior in Duchenne muscular dystrophy
(HealthDay)—In a head-to-head study of mineralocorticoid receptor antagonists, spironolactone was found to be noninferior to eplerenone for slowing the progression of heart damage in boys with Duchenne muscular dystrophy (DMD), according to a study published in the Oct. 1 issue of the Journal of the American Heart Association. Subha V. Raman, M.D., from The Ohio State...
Discovery points to innovative new way to treat Duchenne muscular dystrophy
Researchers at The Ottawa Hospital and the University of Ottawa have discovered a new way to treat the loss of muscle function caused by Duchenne muscular dystrophy in animal models of the disease. As reported in Cell Stem Cell, the team restored muscle stem cell function that is impaired in Duchenne muscular dystophy, resulting in...
Promising new therapy spares muscle loss in Duchenne muscular dystrophy
A promising therapy for Duchenne muscular dystrophy (DMD) developed by University at Buffalo researchers is moving closer to use in humans. The peptide the new drug is based on was originally found in the venom produced by the type of tarantula that Sachs is holding here. Credit: University at Buffalo Published in July in Neuromuscular Disorders, the new UB research demonstrates that the novel...
Archived drug prevents Duchenne Muscular Dystrophy muscle loss in mice
A drug that showed promise in clinical trials for Duchenne Muscular Dystrophy (DMD) decades ago is back under the microscope and has now been shown to reduce muscle wasting in mice. DMD is an incurable, fatal disease that affects mainly boys, at a rate of one in 5,000 globally. Sufferers are usually wheelchair-bound by early adolescence....
Cell therapy improves heart function, upper limb strength in Duchenne muscular dystrophy
After boys and young men with Duchenne muscular dystrophy received cardiac progenitor cell infusions, medical tests indicated that the patients’ hearts appeared improved, results from a new study show. Patients in the study also scored higher on arm strength tests after receiving the cell infusions. Results from the HOPE Duchenne randomized clinical trial of 25...
Brothers with rare terminal muscle disorder fear their future after the FDA denies the drug that keeps them walking
Elliot Johnson, 14, and Henry, 11, both suffer from Duchenne muscular dystrophy It is a rare form of the disease that causes muscle weakness, eventually leading to complete loss of mobility with a mortality rate of around 25 The brothers have been part of a clinical trial with a drug that has shown profound improvements and...
Gene transfer corrects severe muscle defects in mice with Duchenne muscular dystrophy
Duchenne muscular dystrophy is a rapidly progressive disease that causes whole-body muscle weakness and atrophy due to deficiency in a protein called dystrophin. Researchers at the University of Missouri, National Center for Advancing Translational Sciences, University of Washington, and Solid Biosciences, LLC, have developed a new gene transfer approach that uses an adeno-associated virus vector...
Heart drug improves or stabilizes heart function in Duchenne muscular dystrophy
Human heart. Researchers at The Ohio State University Ross Heart Hospital and Nationwide Children’s Hospital have shown early treatment with the heart failure medication eplerenone can improve heart function in young boys with Duchenne muscular dystrophy (DMD) and stabilize heart function in older boys with the disease. The results of their study are published...
- 1
- 2