by Emily LeClerc, University of Wisconsin-Madison
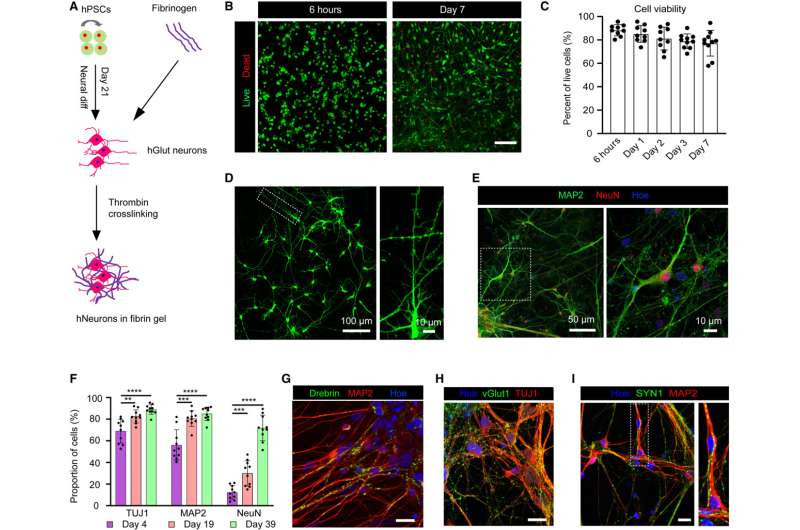
Survival and differentiation of hPSC-derived neurons in fibrin hydrogel. Credit: Cell Stem Cell (2024). DOI: 10.1016/j.stem.2023.12.009
A team of University of Wisconsin–Madison scientists has developed the first 3D-printed brain tissue that can grow and function like typical brain tissue.
It’s an achievement with important implications for scientists studying the brain and working on treatments for a broad range of neurological and neurodevelopmental disorders, such as Alzheimer’s and Parkinson’s disease.
“This could be a hugely powerful model to help us understand how brain cells and parts of the brain communicate in humans,” says Su-Chun Zhang, professor of neuroscience and neurology at UW–Madison’s Waisman Center. “It could change the way we look at stem cell biology, neuroscience, and the pathogenesis of many neurological and psychiatric disorders.”
Printing methods have limited the success of previous attempts to print brain tissue, according to Zhang and Yuanwei Yan, a scientist in Zhang’s lab. The group behind the new 3D-printing process describes their method in the journal Cell Stem Cell.
Instead of using the traditional 3D-printing approach, stacking layers vertically, the researchers went horizontally. They situated brain cells, neurons grown from induced pluripotent stem cells, in a softer “bio-ink” gel than previous attempts had employed.
“The tissue still has enough structure to hold together but it is soft enough to allow the neurons to grow into each other and start talking to each other,” Zhang says.
The cells are laid next to each other like pencils laid next to each other on a tabletop.
“Our tissue stays relatively thin and this makes it easy for the neurons to get enough oxygen and enough nutrients from the growth media,” Yan says.
Researchers produce the first 3D-printed functional human brain tissue
Credit: Cell Stem Cell (2024). DOI: 10.1016/j.stem.2023.12.009
The results speak for themselves—which is to say, the cells can speak to each other. The printed cells reach through the medium to form connections inside each printed layer as well as across layers, forming networks comparable to human brains. The neurons communicate, send signals, interact with each other through neurotransmitters, and even form proper networks with support cells that were added to the printed tissue.
“We printed the cerebral cortex and the striatum and what we found was quite striking,” Zhang says. “Even when we printed different cells belonging to different parts of the brain, they were still able to talk to each other in a very special and specific way.”
The printing technique offers precision—control over the types and arrangement of cells—not found in brain organoids, miniature organs used to study brains. The organoids grow with less organization and control.
“Our lab is very special in that we are able to produce pretty much any type of neurons at any time. Then we can piece them together at almost any time and in whatever way we like,” Zhang says. “Because we can print the tissue by design, we can have a defined system to look at how our human brain network operates. We can look very specifically at how the nerve cells talk to each other under certain conditions because we can print exactly what we want.”
More information: Yuanwei Yan et al, 3D bioprinting of human neural tissues with functional connectivity, Cell Stem Cell (2024). DOI: 10.1016/j.stem.2023.12.009
Provided by University of Wisconsin-Madison

Leave a Reply