by Serena Gordon, Healthday Reporter As hospitals give more and more COVID-19 patients albuterol to help them breathe, people with asthma may have a hard time getting an inhaler. The American College of Allergy, Asthma and Immunology (ACAAI) said some areas of the United States are experiencing shortages of albuterol inhalers, and the shortage may...
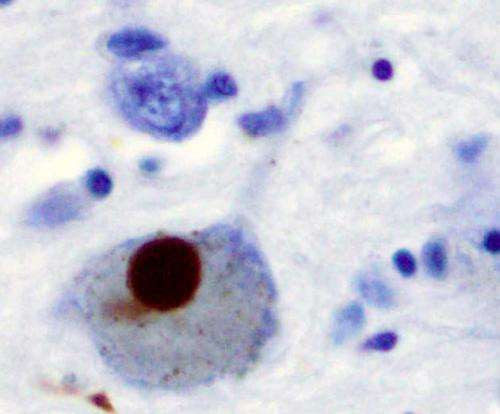
Long-term use of isradipine does not slow Parkinson’s progression
by American College of Physicians Long-term treatment with immediate-release isradipine does not seem to slow the clinical progression of early-stage Parkinson’s disease. Findings from a multicenter, randomized, parallel-group, double-blind, placebo-controlled trial are published in Annals of Internal Medicine. Despite multiple prior studies, there are no proven strategies for slowing the progression of Parkinson’s disease. Isradipine,...
Individuals taking class of steroid medications at high risk for COVID-19
JCEM Editors examine pandemic’s impact on endocrine patients in editorial THE ENDOCRINE SOCIETY WASHINGTON–Individuals taking a class of steroid hormones called glucocorticoids for conditions such as asthma, allergies and arthritis on a routine basis may be unable to mount a normal stress response and are at high risk if they are infected with the virus...
Liraglutide can help adolescents with obesity manage their weight
THE ENDOCRINE SOCIETY WASHINGTON–Liraglutide 3.0 mg, approved by the United States Food and Drug Administration (FDA) as an adjunct to a reduced-calorie diet and increased physical activity to help adults with obesity manage their weight, appears to help adolescents too, according to an industry-sponsored randomized controlled trial. The study was accepted for presentation at ENDO...
Trump calls it a COVID-19 fix. Now lupus patients can’t get their drug
by Lisa Gutierrez Before Aisha Kelley headed to the pharmacy last week, she heard from a fellow lupus patient that she might have trouble getting her prescription filled. Her medication, hydroxychloroquine, sold by the brand name Plaquenil, keeps her body from turning against her. It is considered the most important drug for lupus patients, but...
First FDA-approved drug for thyroid eye disease effective regardless of age, gender
Teprotumumab, the first FDA-approved medicine for thyroid eye disease, provides significant improvement in eye bulging, regardless of patient gender, age or smoking status, according to a study accepted for presentation at ENDO 2020, the Endocrine Society’s annual meeting, and publication in a special supplemental section of the Journal of the Endocrine Society. Though it is...
Cancer treatment with immune checkpoint inhibitors may lead to thyroid dysfunction
by The Endocrine Society Thyroid dysfunction following cancer treatment with new treatments called immune checkpoint inhibitors is more common than previously thought, according to research that was accepted for presentation at ENDO 2020, the Endocrine Society’s annual meeting, and will be published in a special supplemental section of the Journal of the Endocrine Society. Cancer...
Three non-invasive methods used to predict who has NASH agree only about 20% of the time
by The Endocrine Society Researchers and clinicians have been trying to find a way to diagnose nonalcoholic steatohepatitis (NASH) without taking a liver tissue biopsy, but according to new research, formulas that aim to predict NASH based on risk factors do not agree with each other and their accuracy varies. The study was accepted for...
Hypothyroidism patients cite effectiveness in choosing alternative to standard therapy
by The Endocrine Society Three in four hypothyroidism patients who chose desiccated thyroid extract (DTE) over the standard therapy said this option was more effective than other thyroid hormone medications, according to an analysis of comments in online patient forums accepted for presentation at ENDO 2020, the Endocrine Society’s annual meeting, and publication in a...
FDA wants heartburn meds off the market due to contamination
by Matthew Perrone This Sept. 30, 2019 file photo shows a box of Zantac tablets at a pharmacy in Miami Beach, Fla. On Wednesday, April 1, 2020, U.S. health regulators are telling drugmakers to immediately pull their popular heartburn drugs off the market after determining that a contamination issue with the medications poses a greater...