JULY 2, 2024
by Peter MacCallum Cancer Centre
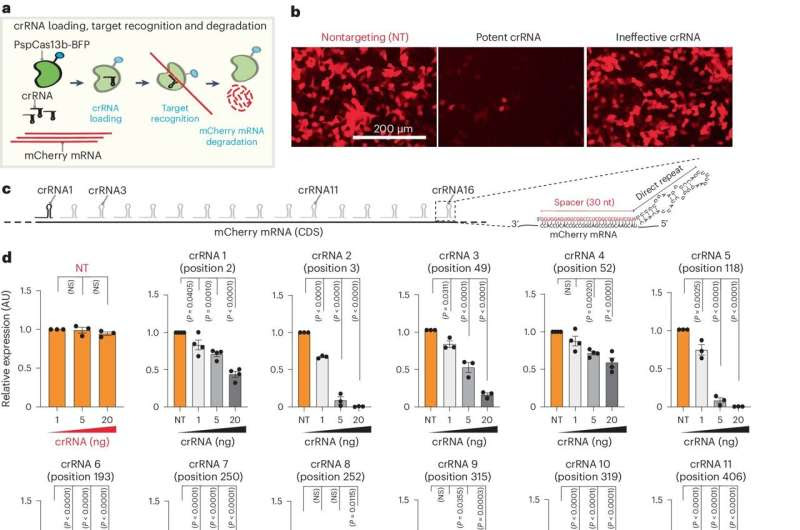
The efficiency of PspCas13b silencing is highly variable between crRNAs. Credit: Nature Structural & Molecular Biology (2024). DOI: 10.1038/s41594-024-01336-0
Peter Mac researchers have taken the first step towards designing rapid personalized cancer treatments by ‘cutting out’ disease-causing RNA.
The findings, published in Nature Structural & Molecular Biology, demonstrate how an innovative technology called CRISPR, previously used to fight viruses like COVID-19, can be adapted to target and destroy other disease-causing genes, including cancer genes.
Dr. Mohamed Fareh, who led the study alongside Dr. Wenxin Hu and their colleagues in the Trapani Lab, said this technology lays the foundation for bespoke treatments tailored to each patient.
“DNA is the blueprint for every cell in the body, but RNA acts as a messenger, carrying information from DNA to produce proteins essential for healthy cells, cancer cells, or pathogenic viruses,” he said.
“Cancer is often driven by abnormal RNA and targeting these ‘harmful RNAs’ is akin to cutting off the cancer’s supply chain. We knew that targeting pathogenic RNA could be a game-changer in fighting diseases like cancer; we just lacked tools that could do so with high precision and efficacy.
“In the future, we hope to build on this knowledge to create successful, personalized cancer treatments.”
Cas9 is a traditional protein used in CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats), a genome editing technology that is used to target genes that cause human diseases.
Cas9 cuts like a pair of scissors at specific locations to delete disease-causing sections of DNA. However, Cas9 can mistakenly cut healthy DNA in the process, which limits its utility in medicine.
To overcome the limitations of Cas9, Peter Mac researchers focused on a different CRISPR protein, Cas13b. The study found this protein cuts RNA with high precision without harming DNA.
Dr. Fareh highlighted that the team has been re-engineering Cas13b tools for over five years. They initially engineered a version of Cas13b to silence the COVID-19 virus just after the beginning of the pandemic. However, this initial design was labor-intensive, inefficient, and error-prone.
In their latest study, the researchers used a method called Single-Base Tiled screening and computer analysis to figure out how to make Cas13b more effective in cutting a target RNA in lab-grown human cells. By elucidating new design parameters, they upgraded Cas13b design to eliminate any RNA, including cancer RNA.
“We are excited about this research because we have solved the problem of how to make this technology precise and efficient at finding and eliminating abnormal RNA without cutting any healthy RNA in the human cell,” Dr. Fareh said.
“To make this technology accessible to broader scientific and medical communities, we have also created an online tool that accurately predicts the correct sequences to cut. We believe this online tool will enable the targeting of a wide range of disease-causing RNA including those in cancer.”
RNA is enjoying a renaissance in both biology and medicine due to the efficacy of mRNA-based COVID-19 vaccines and holds tremendous promise for treating many diseases, including cancer.
More information: Wenxin Hu et al, Single-base tiled screen unveils design principles of PspCas13b for potent and off-target-free RNA silencing, Nature Structural & Molecular Biology (2024). DOI: 10.1038/s41594-024-01336-0
Journal information: Nature Structural & Molecular Biology

Leave a Reply