JULY 23, 2024
by Sanford-Burnham Prebys
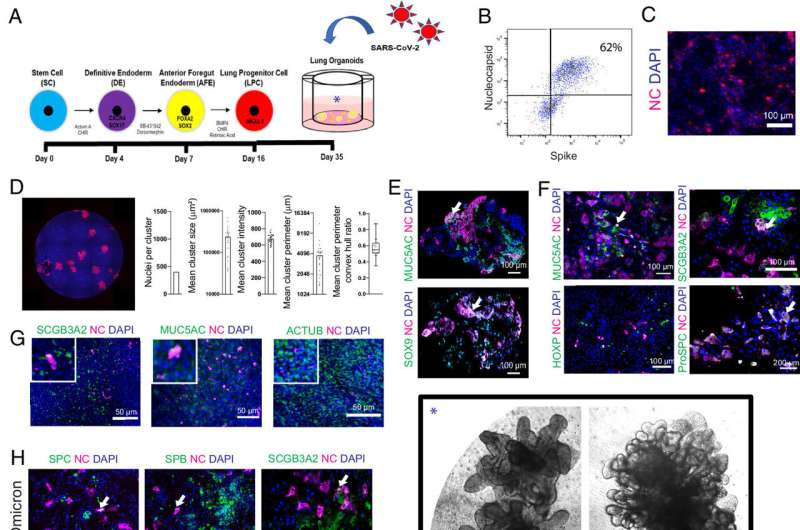
hiPSC-derived LOs are susceptible to infection with multiple strains of SARS-CoV-2, validated in primary human lung epithelia. Credit: Proceedings of the National Academy of Sciences (2024). DOI: 10.1073/pnas.2408109121
Scientists at Sanford Burnham Prebys, University of California San Diego and their international collaborators have reported that more types of lung cells can be infected by SARS-CoV-2 than previously thought, including those without known viral receptors. The research team also reported for the first time that the lung is capable of independently mustering an inflammatory antiviral response without help from the immune system when exposed to SARS-CoV-2.
This work is especially timely, as cases of COVID-19 are on the rise in the scientists’ hometown of San Diego during a summertime spike. Looking beyond the region, more than half of the states in the country have reported “very high” or “high” levels of infection, according to the Centers for Disease Control and Prevention.
“Headlines have come and gone, but SARS-CoV-2 and COVID-19 have never left,” says Evan Snyder, MD, Ph.D., director of the Center for Stem Cells and Regenerative Medicine and a professor in the Human Genetics Program at Sanford Burnham Prebys. “And neither have the scientists studying it.”
“While many people experience mild or moderate symptoms, COVID-19 still kills,” adds Sandra Leibel, MD, MSc, a neonatologist at Rady Children’s Hospital-San Diego and an associate professor of Pediatrics at UC San Diego School of Medicine. “This virus is here to stay, and we need to learn everything we can about it so we can improve treatment and prevention.”
Leibel, Snyder and their collaborators reported their new discoveries about SARS-CoV-2 and COVID-19 in a paper published in Proceedings of the National Academy of Sciences. The scientists used a technique to transform cells taken from patients into cells resembling stem cells. These embryonic-like cells—known as induced pluripotent stem cells (iPSCs)—can then be turned into other types of human cells. The team caused them to develop into a grouping of various lung cell types in a pattern that mimics the human lung at a smaller scale.
“With most models for studying respiratory infections, you can’t isolate a specific cellular response because you have all the immune system cells rushing in to help deal with the invaders,” notes Snyder.
“Using our lung organoids or ‘mini lungs,’ another benefit is that we can choose the sex of the cells, so we’re not just studying male-dominant or female-dominant lung tissue,” adds Leibel. “This is important, as we know that the lung responds differently during disease if you’re a female or a male.”
In addition, the team could make iPSCs from patients of different racial and ethnic groups to try to understand the known disparity in this and other diseases—both in terms of susceptibility to infection, severity of the consequences, and responsiveness to various medications.
The scientists observed that SARS-CoV-2 was able to acutely infect many previously undescribed cell types in the mini lungs. This held true when testing different strains of SARS-CoV-2, although it was clear that certain strains were more effective at infecting specific cell types.
“People used to say that SARS-CoV-2 only infects cells with certain receptors, especially those with the ACE2 receptor known to interact with the infamous SARS-CoV-2 spike protein,” says Snyder. “We demonstrated that when a direct entry point was unavailable, the virus just punches through the cell membrane instead.”
“With the delta variant having produced more severe symptoms, and the omicron variant being less deadly but more contagious, we hypothesized that delta may prefer the alveolar cells deeper in the lungs, while omicron sticks more to the upper airways,” adds Leibel. “While all strains were capable of infecting many lung cell types, we did see a distinct preference for these strains, as predicted.”
In fact, as the strains changed over time, the scientists could see what was reported in patients as the character of the pandemic changed. Earlier strains such as delta caused more deadly pneumonias because they affected lower lung cells. Later strains like omicron affected more upper lung cells and led to clinicians seeing fewer pneumonias and more airway problems and sore throats. So, the mini-lung system may help the team predict patient outcomes.
In addition to demonstrating how the virus infects cells previously thought to be safe, the scientists also found a way to block the virus’s unexpected flanking maneuver. The team demonstrated that apilimod—a drug currently being studied for potentially treating cancer, ALS, dementia and various viral infections—effectively blocked the backdoor entry of SARS-CoV-2 into cells lacking traditional entry points.
“Our data suggest that apilimod could be an adjunct therapy given early on to slow down the infection and enhance the effectiveness of other medicines and the innate immune response,” says Snyder.
In another surprising result, Leibel, Snyder and team discovered that the mini lungs have their own intrinsic “first response” system in reaction to sensing SARS-CoV-2. Even though the mini lungs lack any connection to an immune system, this study shows that lung cells can initiate many of the same biologic and cell signaling changes in response to a viral threat that are observed when the immune system is present.
“We found that lung cells are capable of autonomously reacting to infection immediately while also subsequently calling for reinforcements from the immune system,” adds Snyder.
“We showed that it’s not just the immune cells that are becoming over-activated and secreting too much of the pro-inflammatory cytokines that contribute to severe cases of COVID-19,” says Leibel. “The lung cells do this as well.”
The scientists learned that this inherent antiviral response system in the mini lungs was orchestrated by an unlikely source: one of the four proteins that mix with fats to form a soapy substance in the lungs’ air sacs that helps keep them open as we breathe. This detergent-like substance is called surfactant, and its constituent protein surfactant protein B (SP-B) turned out to be the most important player in the mini lungs’ attempts to ward off SARS-CoV-2. No prior research had suggested that SP-B played any cellular signaling roles.
“When we tested mini lungs genetically engineered to not express SP-B, we saw triple the number of cells infected with SARS-CoV-2,” says Leibel. “When we followed that up by treating these engineered mini lungs with SP-B in a similar way to how premature newborns with surfactant deficiency are treated, we noted a reduction in viral infectivity.”
“These findings suggest not just one but two potential novel drug applications with the possible clinical use of surfactant early in COVID-19 cases,” adds Snyder. “This is important, as we only have two current proven antiviral drugs in Paxlovid and remdesivir.”
The team plans to follow up these findings with studies to determine exactly how surfactant is so effective at protecting cells against viral invasion. Also, they are investigating whether a rapid test for SP-B as well as certain characteristic pro-inflammatory cytokines may help quickly determine which people are at greater risk of more severe forms of COVID-19.
Leibel adds, “This would help people make more informed decisions about traveling and attending public events during spikes of COVID-19, and it also would help physicians tailor treatments for those at an increased risk of serious disease.”
More information: Sandra L. Leibel et al, A therapy for suppressing canonical and noncanonical SARS-CoV-2 viral entry and an intrinsic intrapulmonary inflammatory response, Proceedings of the National Academy of Sciences (2024). DOI: 10.1073/pnas.2408109121
Journal information: Proceedings of the National Academy of Sciences
Provided by Sanford-Burnham Prebys

Leave a Reply