JULY 25, 2024
by King’s College London
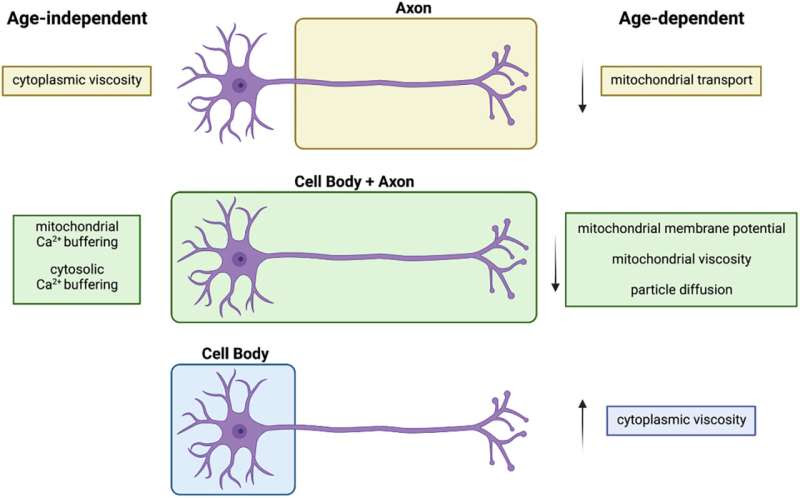
Model of mitochondrial homeostasis and cytoplasmic viscosity during aging. Credit: Aging Cell (2024). DOI: 10.1111/acel.14250
A team from King’s College London and collaborators have pinpointed how different areas of the neuron age differently, with a potential knock-on effect on healthy cell function and the development of neurodegenerative conditions. The paper is published in the journal Aging Cell.
A neuron is a nerve cell that sends and receives information from the brain and communicates with the rest of the body. Unlike other cells, neurons do not replicate and so must find other methods of keeping healthy and operational.
One method of doing so is mitochondrial trafficking, where the parts of the cell responsible for making energy are transported from the central body of the neuron (soma) to its axon, a tail-like structure that transmits electrical and chemical signals to other cells. The rate of mitochondrial trafficking decreases during aging and neurodegenerative illnesses such as ALS and AD and is considered a litmus test for further degeneration in the brain.
However, it has been unclear to what extent the decline of mitochondrial transport and function observed during aging are coupled, and mechanistically how this process works, until now.
The researchers used fluorescence to measure the viscosity of neuronal cytoplasm, the fluid enclosed by the cell membrane of the neuron, in mice. They discovered that as the mice aged, the viscosity of the cytoplasm in the soma increased, but not in the cytoplasm of the axon.
They theorize that mitochondria cannot be exported from the cell body into the axons as they get trapped in the viscous soma, negatively affecting cell health.
By comparing cytoplasmic viscosity in healthy patients of a similar age, the researchers suggest that, in future, changing viscosity could be potentially used as a warning sign for neurodegenerative disease.
Dr. Alessio Vagnoni, Lecturer in Cellular Neuroscience at King’s College London and the study’s leading author said, “Trafficking of cellular components, such as mitochondria, is one of the most effective benchmarks of a healthy neuron—essentially, movement is life and stillness is death.
“Cellular biophysics has been a growing area of interest in neuroscience and previous studies have shown that proteins linked to disease are affected by the biophysical state of the cytoplasm of the neuron.
“By providing a more complete picture of a target area for drugs, our work could even improve pharmaceutical treatments,” says Vagnoni.
“By measuring viscosity in a model of neuronal aging for the first time means we could also start mapping specific age-dependent neurodegenerative diseases to cytoplasmic viscosities, and potentially expand the list of biomarkers for a range of illnesses. Eventually, by providing a more complete picture of a target area for drugs, this could even improve pharmaceutical treatments.”
The team now hope to study how environmental factors like additional illnesses might impact the cytoplasm in the neuron during age, and how this may lead to neurodegeneration.
More information: James N. Sleigh et al, Age‐specific and compartment‐dependent changes in mitochondrial homeostasis and cytoplasmic viscosity in mouse peripheral neurons, Aging Cell (2024). DOI: 10.1111/acel.14250
Journal information: Aging Cell
Provided by King’s College London

Leave a Reply