Pharma
By Isabella Cueto Aug. 9, 2024
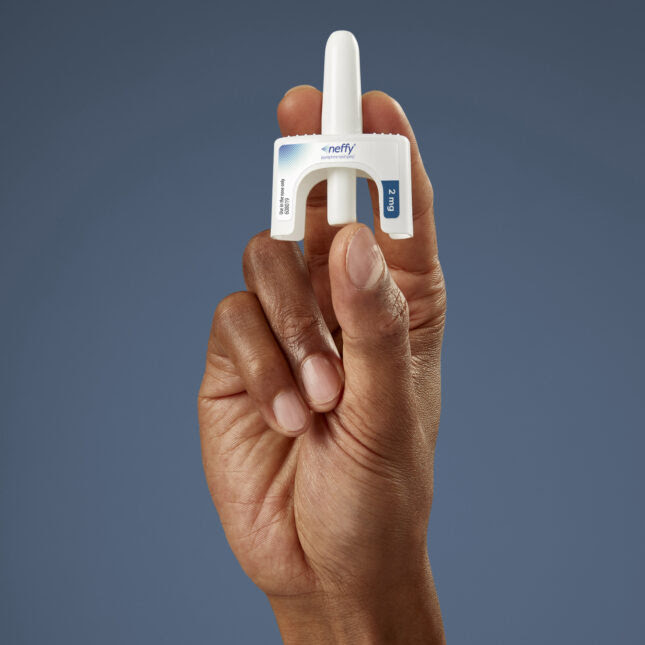
Neffy, the new nasal spray for severe allergic reactions, is approved for use in people 66 pounds and above.
Courtesy ARS Pharma
On Friday, the Food and Drug Administration approved the first needle-free treatment for adults and kids with severe allergic reactions. The approval introduces a competitor from ARS Pharma to older epinephrine products, like the EpiPen.
Neffy, as the product is called, is a nasal spray that delivers the same drug used to treat anaphylaxis. It is ARS’ flagship product. Friday’s approval comes after the FDA stalled neffy’s approval in September, asking the drugmaker for additional data.
At the time, the company was being asked to conduct a repeat-dose study of neffy to show it worked as well as available epinephrine products. (Viatris, the maker of the EpiPen, also filed a petition asking for more data, Endpoints reported.) Much of the data ARS submitted last year was from people who were healthy or only had mild allergies (not anaphylaxis), there was limited safety data, no formal clinical efficacy trial, and results varied across trials.
Related: A needle-free anaphylaxis treatment runs into an FDA roadblock
The 2-milligram dose of neffy was approved for anaphylaxis in adults and children who weigh more than 66 pounds. One spray goes in a single nostril, and the second can be used as backup if the first fails to curb anaphylaxis. Experts recommend seeking out medical care during an episode of anaphylaxis, even if a person has taken epinephrine.
The review process for epinephrine is based on surrogate measures of efficacy, such as how well neffy raises a person’s blood pressure (a necessary step for calming an allergic reaction). Epinephrine has been widely used since before the FDA existed, so its approval process is different from that of newer drugs.
Neffy’s approval was based on four studies that included 175 healthy adults without anaphylaxis. Concentrations of epinephrine in their blood after using neffy were comparable to those after using an auto-injector pen, according to a new release from the FDA.
An epinephrine nasal spray has been difficult to develop because the drug is not well-absorbed through the nose, said ARS CEO Richard Lowenthal, a 25-year veteran in drug development who started his career as a new drug reviewer at the FDA.
ARS presents neffy as a smaller and more easily administered rival to auto-injectors which, for decades, have been the only quick treatment option for people with severe allergies. Epinephrine, which is the same thing as adrenaline, has been used for a century to treat anaphylaxis. However, existing treatments have run into supply, cost, and design issues neffy hopes to fix.
Lowenthal told STAT in September that a pair of neffy would not cost more than $199, even for people who are uninsured or have high-deductible health insurance plans. For most people with commercial insurance, ARS plans to use coupons to drive copays down to $25 per two-pack.
He also said he doesn’t expect to encounter the same supply issues that have plagued other epinephrine products. Since 2017, there have been shortages of the EpiPen, and other auto-injectors have been pulled from the market over quality control problems. Lowenthal said it’s the complicated design of the pens that has been a problem. Epinephrine itself is available, and neffy will be using a simple spray device, the same one used for overdose reversal medication Narcan and several other products on the market. Neffy’s nasal spray containers are about the size of a tea bag, and deliver a single dose into the nose with a one-handed pump.
ARS’ research suggests children as young as 12 years old can use neffy by themselves (the drugmaker chose the name to be kid-friendly). “It doesn’t matter if you dose it upside down, right side up, at an angle — doesn’t matter. It fires exactly the same way in every direction,” Lowenthal said. And ARS has designed a clip-on carrying case reminiscent of an AirPods case to keep the doses in.
Neffy could also be stored at up to 122 degrees Fahrenheit for up to three months, meaning users don’t need to worry about the drug going bad if they leave it in a hot car for an afternoon, Lowenthal said. That pushes the temperature stability further than auto-injectors, he said.
Reported side effects of neffy include: throat irritation, headache, nasal discomfort, fatigue, tremor, runny or itchy nose, sneezing, abdominal pain, gum pain, numbness in the mouth, nasal congestion, dizziness, nausea and vomiting. People with nasal polyps or a history of nasal surgery may not absorb neffy well, and those with allergic reactions to sulfite should talk to a doctor before using the spray.
Lack of access to epinephrine — because of cost or supply, or insurance coverage — can put people with severe allergic reactions in risky situations, experts say. While deaths from anaphylaxis are rare, delaying treatment means the allergic reaction will worsen and require hospitalization.
Access issues widen health disparities. Already, data suggest there are significant differences in food allergy prevalence by socioeconomic status. And Black, Hispanic, and Asian children have higher rates of severe allergic reactions, allergies to multiple foods, and related emergency visits, Ruchi Gupta, a pediatrician and founding director of the Center for Food Allergy & Asthma Research at Northwestern University’s Feinberg School of Medicine and Lurie Children’s Hospital of Chicago found in her research. (The top nine allergens are peanuts, tree nuts, shellfish, finned fish, milk, eggs, soy, wheat, and sesame.)
ARS will next look to expand the patient pool to children of lower weights. The drugmaker also told STAT it was conducting trials to see if neffy can work for people with chronic hives or persistent asthma.
STAT’s coverage of chronic health issues is supported by a grant from Bloomberg Philanthropies. Our financial supporters are not involved in any decisions about our journalism.
About the AuthorReprints
Isabella Cueto
Chronic Disease Reporter
Isabella Cueto covers the leading causes of death and disability: chronic diseases. Her focus includes autoimmune conditions and diseases of the lungs, kidneys, liver (and more). She writes about intriguing research, the promises and pitfalls of treatment, and what can be done about the burden of disease.
[email protected]
@isabellacueto
linkedin.com/in/isabellacueto/

Leave a Reply