September 5, 2024
by Eddy Duryea, University of Central Florida
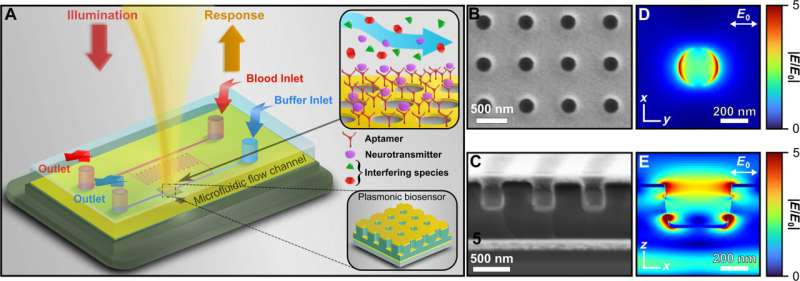
Description of plasmonic biosensor platform. (A) Schematic of integrated plasmonic biosensor platform. Top inset shows specificity of surface-functionalized DNA aptamer in presence of other interfering species, where the aptamer binds selectively to dopamine. Bottom inset shows a cartoon representation of the plasmonic biosensor. (B and C) Scanning electron microscopy (SEM) image of the biosensor showing surface (B) and cross-sectional (C) view. (D and E) Finite-difference time-domain (FDTD)–predicted local electric near-field enhancement at λ = 842 nm, 2 nm above the surface (D) and cross section (bottom). Credit: Science Advances (2024). DOI: 10.1126/sciadv.adp7460
Dopamine, a neurotransmitter in our brains, not only regulates our emotions but also serves as a biomarker for the screening of certain cancers and other neurological conditions.
University of Central Florida researchers, led by UCF NanoScience Technology Center Professor Debashis Chanda, have developed an integrated optical sensor capable of detecting dopamine directly from an unprocessed blood sample. This sensor may serve as a low-cost and efficient screening tool for various neurological conditions and cancers, ultimately providing better outcomes for patients.
The study is published in the journal Science Advances.
“This plasmonic biosensor is extremely sensitive to low concentrations of biomolecules, which makes them a promising platform for specialized assays and point of care applications in remote locations,” says Chanda, the study’s principal investigator who also has appointments in UCF’s Department of Physics and CREOL, the College of Optics and Photonics.
“In this work, we demonstrated an all-optical, surface-functionalized plasmonic biosensing platform for the detection of low concentrations of neurotransmitter dopamine directly from diverse biological samples, which includes protein solutions, artificial cerebrospinal fluid, and unprocessed whole blood.”
Neurotransmitters play a vital role in regulating neural function and overall well-being in humans and animals, necessitating a harmonious balance of neurological hormones for optimal bodily function, Chanda says. Dopamine serves as a crucial transmitter, as it exerts significant influence over cognitive processes such as motor functions or emotions like happiness or pleasure.
Disruptions in dopamine levels are closely linked with various neurodegenerative disorders such as Parkinson’s disease and Alzheimer’s disease, neurodevelopmental conditions like attention deficit hyperactivity disorder and Tourette’s Syndrome, and psychological disorders such as bipolar disorder and schizophrenia, Chanda says.
Deviations from normal dopamine levels can also serve as an important diagnostic marker for certain types of cancers. The precise and dependable measurement of dopamine concentrations is of the utmost importance for advancing pharmaceutical research and medical therapies, Chanda says.
How it works
The plasmonic sensor is made of a tiny gold pattern that causes electrons to move together in waves. These waves, called plasmons, become stronger with a special optical setup. When a new molecule enters the sensor’s environment, it changes how the electrons move, which affects how light is reflected from the sensor. This change in reflection helps detect the presence of the molecule.
Unlike traditional biosensors that rely on biological elements like antibodies or enzymes, this UCF-developed device uses a specially designed aptamer—a synthetic DNA strand—to precisely detect dopamine. This approach not only makes the sensor more cost-effective and easier to store, but it also allows the device to detect dopamine directly from unprocessed blood without any preparation. This breakthrough could be particularly valuable in areas with limited medical resources, as it simplifies the detection process and opens the door for diagnosing other conditions using the same technology.
Researchers were able to target specific molecules by coating the sensor’s active area with an aptamer specifically created to latch onto a particular biomarker with great accuracy.
The study’s results highlight the potential of plasmonic “aptasensors” using aptamers to sense for developing rapid and accurate diagnostic tools for disease monitoring, medical diagnostics, and targeted therapies, the researchers say.
“There have been numerous demonstrations of plasmonic biosensors but all of them fall short in detecting the relevant biomarker directly from unprocessed biological fluids, such as blood,” says Aritra Biswas, Ph.D., the lead author of the paper.
The new research builds upon the team’s previous work developing a dopamine detector by replacing cerium oxide nanoparticles with DNA-based aptamers, enhancing the sensor’s selectivity and expanding its applicability to detect dopamine directly in diverse biological samples without needing prior sample preparation.
“This concept can be further explored in the detection of different biomolecules directly from unprocessed blood, such as proteins, viruses, DNA,” says Chanda. “There may be great interest in developing countries where access to analytical laboratories is limited.”
More information: Aritra Biswas et al, Nanoplasmonic aptasensor for sensitive, selective, and real-time detection of dopamine from unprocessed whole blood, Science Advances (2024). DOI: 10.1126/sciadv.adp7460
Journal information: Science Advances
Provided by University of Central Florida

Leave a Reply