September 23, 2024
by Delthia Ricks , Medical Xpress
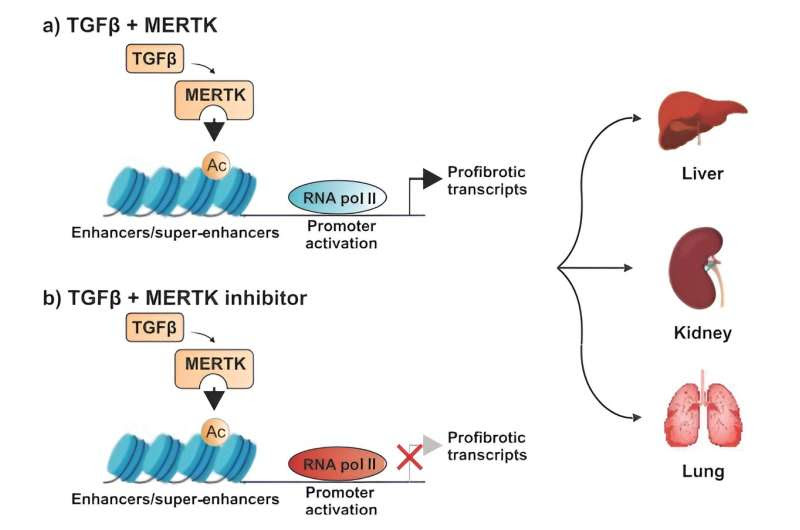
Model of MERTK mechanisms for regulating TGFβ core fibrotic pathway across various organs. Credit: Science Translational Medicine (2024). DOI: 10.1126/scitranslmed.adj0133
Medical science has long been on the hunt for a deeper understanding of the devastating scarring of the body’s organs known as fibrosis, which leads to irrevocable loss of function.
Fibrosis refers to aberrant connective tissue that accumulates in organs impairing their ability to function properly, a process triggered by chronic inflammatory activity. These inflammatory processes can be induced by persistent infection, chemical insults or severe tissue injury. For some patients, the underlying cause of their fibrotic disease remains stubbornly elusive. Fibrotic scarring not only robs patients of healthy organ function, in some instances it steals their lives.
Scar tissue can damage any of the body’s major organs, emerging in the lungs as idiopathic pulmonary fibrosis, or in the liver as fatty liver disease. Kidney and cardiac fibrosis are conditions that can be marked by severe organ impairment. Explained another way, a heart attack can cause scar tissue build up—myocardial fibrosis—but the fibrotic region lacks the contractile activity of healthy heart tissue. Worse, there aren’t many therapies that can durably treat or reverse organ fibrosis, making the search for potential therapies a top research priority.
Now, medical researchers in Australia are finding new clues about fibrosis and how the damaging condition is sustained by a positive feedback loop in organs marked by pervasive fibrotic scarring.
By isolating and defining the molecular activity of the feedback loop, Dr. Ziyan Pan and colleagues of the Westmead Institute for Medical Research near Sydney, have opened a new window of understanding about fibrosis by pinpointing a promising target for drug therapy.
In a study published in Science Translational Medicine, Pan and a team of collaborators underscored that isolating the positive feedback loop was critical because it helped explain the persistence and sometimes deadly role fibrosis can play in organ damage.
Tissue fibrosis, Pan and colleagues write, underlies many chronic diseases that are difficult to treat. And while the cytokine known as transforming growth factor-β—TGFβ—regulates fibrosis, it is also involved in other critical biological processes, making it an improbable target for pharmaceutical development.
“TGFβ drives fibrosis and disease progression in a number of chronic disorders, but targeting this ubiquitously expressed cytokine may not yield a viable and safe antifibrotic therapy,” Pan writes in the journal.
“We sought to identify alternative ways to inhibit TGFβ signaling using human hepatic stellate cells and macrophages from humans and mice in vitro, as well as mouse models of liver, kidney, and lung fibrosis,” Pan continued.
Given that TGFβ is problematic as a potential drug target, Pan and colleagues hunted down a different, more durable one. During their study of mouse models of liver, kidney, and lung fibrosis, the researchers found that the organs displayed very high levels of an enzyme known as MERTK.
They also found that liver biopsy samples from patients with fibrotic fatty liver disease also harbored high levels of MERTK, especially samples from patients with markedly advanced stages of fibrosis.
What the team did next laid the groundwork for a potential treatment strategy against fibrosis regardless of the organ the disease attacks.
Turning again to mouse models of liver, kidney and lung fibrosis, Pan and the other scientists found that MERTK also induced TGFβ expression and drove TGFβ signaling, resulting in the positive feedback loop that promoted fibrosis in cultured cells. As part of their study, the researchers found a way to blunt MERT—stopping TGFβ signaling and the ongoing process of fibrosis.
“MERTK increased transcription of genes regulating fibrosis by modulating chromatin accessibility and RNA polymerase II activity,” Pan wrote. “In each of the three mouse models, disrupting the fibrosis-promoting signaling loop by reducing MERTK expression reduced organ fibrosis.”
Scientists introduced an experimental drug to fibrotic cells, a MERTK inhibitor called UNC569. The investigational pharmaceutical reduced fibrosis in the liver, kidneys, and lungs of mouse models when given early in the disease process. To the surprise of Pan and the team of scientists, the experimental drug also reversed liver fibrosis when given to mice with established liver damage.
“Pharmacological inhibition of MERTK reduced fibrosis in these mouse models either when initiated immediately after injury or when initiated after fibrosis was established,” Pan asserted. “Together, these data suggest that MERTK plays a role in modulating organ fibrosis and may be a potential target for treating fibrotic diseases.”
Whether the experimental MERTK inhibitor known as UNC569 becomes a pharmaceutical for human testing is a question that has yet to be addressed. Pan and colleagues have more importantly demonstrated that fibrosis is sustained in tissues by the same positive feedback loop and that the loop can be disrupted pharmaceutically.
“These findings broaden our understanding of the roles of MERTK, but also reveal new regulatory molecules that regulate TGF-β activity during fibrosis,” Pan concluded.
More information: Ziyan Pan et al, Inhibition of MERTK reduces organ fibrosis in mouse models of fibrotic disease, Science Translational Medicine (2024). DOI: 10.1126/scitranslmed.adj0133
Journal information: Science Translational Medicine
© 2024 Science X Network

Leave a Reply