Signalling from the sympathetic nervous system of mice when subjected to stress leads to the depletion of a stem-cell population in their hair follicles. This discovery sheds light on why stress turns hair prematurely grey.
Shayla A. Clark &
Christopher D. Deppmann
It has been said that Marie Antoinette’s hair went completely white on the night before her beheading. This story might be apocryphal, but rapid greying of the hair is now widely referred to as Marie Antoinette syndrome. It is often assumed to be caused by stress — a phenomenon perhaps best exemplified by photographs of heads of state before and after they held office. However, the relative contributions of ageing, genetic factors and stress to greying are not known — in part owing to a lack of mechanistic understanding of the process. Writing in Nature, Zhang et al.1 identify the mechanism governing premature greying in mice that have experienced stress.
The average human scalp has 100,000 hair follicles, and a wide range of hair colours can be found across the human population. Hair colour is determined by cells called melanocytes, which produce different combinations of light-absorbing melanin pigments2. Melanocytes are derived from melanocyte stem cells (MeSCs), which are located in a part of the hair follicle called the bulge3. The normal hair cycle is divided into three stages: hair-follicle regeneration (anagen), degeneration (catagen) and rest (telogen). Melanocyte production begins early in the anagen phase (Fig. 1a). As people age, the pool of MeSCs is gradually depleted — and so pigmented hair becomes ‘salt and pepper’ coloured, and then turns to grey and finally to white after a complete loss of pigment in all hair follicles4.
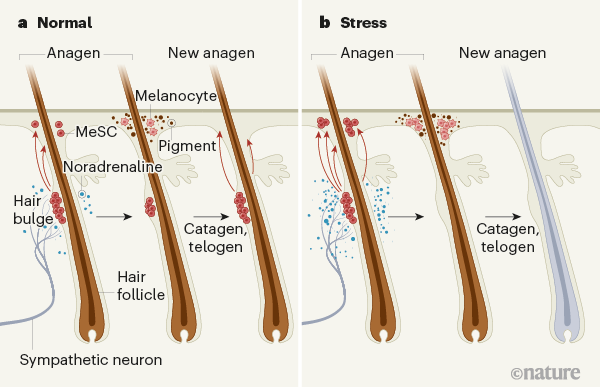
Aside from ageing, there are several factors that bring about premature greying, including dietary deficiencies5, disorders such as alopecia areata or vitiligo6,7, and stress8,9. Zhang et al. set out to test the role of stress in the greying process in mice. They exposed the animals to three different stressors — pain, restraint and a model of psychological stress — during different phases of hair growth. Each stressor caused depletion of MeSCs from the bulge region, eventually leading to the development of patches of white hair.
Prevailing theories posit that stress-induced greying involves hormones (such as corticosterone) or autoimmune reactions10. Zhang and colleagues examined these potential mechanisms, first by preventing corticosterone signalling and next by stressing animals that had compromised immune systems. In both cases, greying occurred after stress, indicating that neither corticosterone nor autoimmune reactions cause MeSC depletion. However, the authors found that MeSCs express β2-adrenergic receptors, which respond to noradrenaline — a neurotransmitter molecule involved in the ‘fight or flight’ response to stress. Loss of this receptor specifically in MeSCs completely blocked stress-induced greying.
Adrenal glands are the main source of circulating noradrenaline. But, surprisingly, the researchers discovered that removing these glands did not prevent greying in response to stress in the mice.

Another source of noradrenaline is the sympathetic nervous system (SNS), which is highly active in response to stress, and which drives the fight-or-flight response. Zhang and colleagues showed that bulge regions are highly innervated by sympathetic neurons, and that ablating the SNS using a neurotoxin molecule, or blocking the release of noradrenaline from sympathetic neurons, prevented stress-induced greying. Next, the authors generated mice in which sympathetic neurons could be acutely activated, and found that overactivation of the SNS in these mice caused greying in the absence of stress. Together, these results indicate that noradrenaline released from active sympathetic neurons triggers MeSC depletion (Fig. 1b). Interestingly, Zhang et al. found that the propensity of an area to turn grey correlates with its level of sympathetic innervation.
Exactly how does sympathetic activity cause depletion of MeSCs from hair follicles? Normally, these stem cells are maintained in a dormant state until hair regrowth is required. However, when the researchers tracked MeSCs labelled with a fluorescent protein, they discovered that MeSC proliferation and differentiation increase markedly under extreme stress or exposure to a high level of noradrenaline. This results in mass migration of melanocytes away from the bulge, and leaves no remaining stem cells. To further confirm this result, the researchers suppressed MeSC proliferation pharmacologically and genetically. When proliferation was dampened, the effects of stress on MeSC proliferation, differentiation and migration were blocked.
Zhang and colleagues’ work raises several questions. For instance, is the mechanism underlying MeSC depletion in response to stress the same as that which causes greying during ageing? Future experiments modulating SNS activity over a longer period would determine whether age-related greying can be slowed or hastened. Perhaps, in the absence of sympathetic signals, MeSCs have the capacity for unlimited replenishment, pointing to a way to delay age-related greying.

Cell umbrella protects stem cells from damage
Are other pools of stem cells similarly susceptible to stem-cell depletion in response to stress, if they or the cells that make up their niche express β2-adrenergic receptors? In support of this idea, haematopoietic stem and progenitor cells (HSPCs), which give rise to blood and immune lineages, reside in a bone-marrow niche that contains stromal cells, and stimulation of those cells by the SNS causes HSPCs to leave their niche11,12. Perhaps, like MeSCs, stress depletes HSPCs — which could partially explain why immune function is impaired in response to chronic stress13,14. Whether this type of relationship extends beyond MeSCs and HSPCs is an open question.
It is fascinating to consider what possible evolutionary advantage might be conferred by stress-induced greying. Because grey hair is most often linked to age, it could be associated with experience, leadership and trust 15. For example, adult male silverback mountain gorillas (Gorilla beringei beringei), which get grey hair on their backs after reaching full maturity, can go on to lead a gorilla troop 16. Perhaps an animal that has endured enough stress to ‘earn’ grey hair has a higher place in the social order than would ordinarily be conferred by that individual’s age.
Connecting the dots between stress, fight or flight, stem-cell depletion and premature greying opens up several avenues for future research. Beyond developing anti-greying therapies, Zhang and colleagues’ work promises to usher in a better understanding of how stress influences other stem-cell pools and their niches.

Leave a Reply