by Fred Hutchinson Cancer Research Center
Millions of Americans live with the pain and physical limitations of arthritis and other inflammatory diseases. Steroids and other drugs can offer relief, but they can also come with a host of serious side effects.
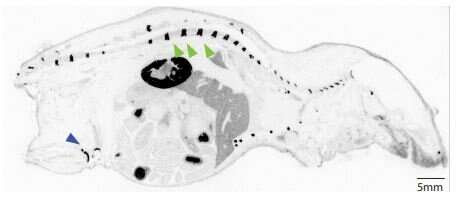
A new study published March 4 in Science Translational Medicine suggests that a scorpion-derived mini-protein could one day help tame the sting of that toxic tradeoff.
Scientists at Fred Hutchinson Cancer Research Center identified a tiny protein in scorpion venom that rapidly accumulates in joint cartilage. Then they linked these mini-proteins with steroids to reverse inflammation in rats with arthritis. The researchers found that the drugs concentrated in the joints, potentially avoiding the body-wide toxicities and infection risks caused by nontargeted steroid treatment.
“For people with multi-joint arthritis, the side effects of controlling the disease can be as bad or worse than the disease itself,” said the project’s senior scientist Dr. Jim Olson, a member of the Clinical Research Division at Fred Hutch. “Steroids like to go everywhere in the body except where they’re needed most. This is a strategy to improve arthritis relief with minimal systemic side effects.”
While the approach is several years away from human patients, it is a promising proof of concept, Olson said.
Olson uses blueprints from nature to discover and develop new drugs. The latest study stemmed from years-long research into what Olson has dubbed optides, short for “optimized peptides.” These tiny proteins are derived from natural organisms such as scorpions, snakes, violets and sunflowers.
“My thought was that these peptides that are in venoms or toxins might have really unique biodistribution in human bodies,” Olson said. “If something is using them for predation, they need to get to certain places rapidly.”
More than a decade ago, Olson discovered a mini-protein found in the deathstalker scorpion that can bind to cancer cells but not healthy ones. He co-founded a company, Blaze Bioscience, in 2010 to develop an experimental dye called Tumor Paint BLZ-100 made from a special, glowing version of the deathstalker’s targeting protein. It is now being tested as a tool for surgeons to precisely illuminate hard-to-see brain tumors.
Once Blaze spun out from Fred Hutch, Olson wondered what other potential drugs might be lurking in nature.
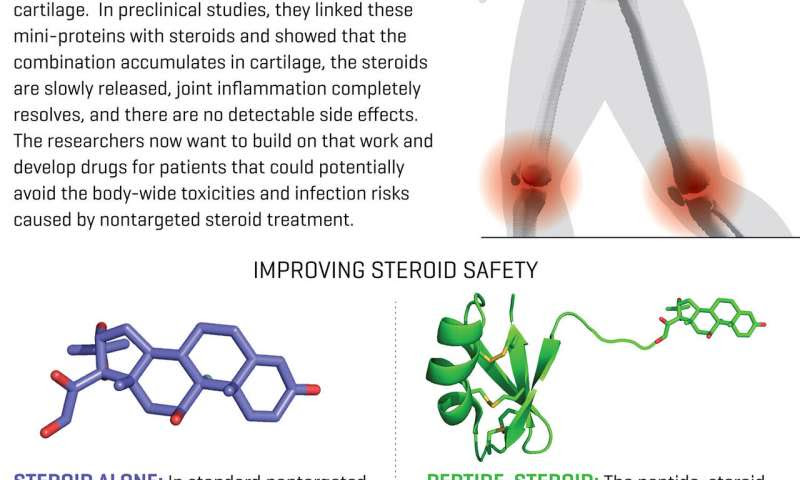
Infographic showing how a future treatment of arthritis could involve using a scorpion-derived mini-protein (a peptide) that delivers arthritis drugs directly to joint cartilage. The approach could potentially improve the safety of arthritis treatment. Credit: Fred Hutchinson Cancer Research Center
Four years ago, Olson and his team were screening dozens of peptides derived from scorpions and spiders. They were hunting for other molecules that could also cross the blood-brain barrier. (Which is an extremely difficult task; the protective barrier is designed to keep most everything out of the brain.) When one peptide appeared to accumulate and linger in cartilage, they instantly realized this could be a treatment for arthritis.
“It really shows the value of playing scientifically and just doing things for the pure joy of learning,” Olson said. “You never know where it’s going to take you. If we could relieve arthritis for millions of people with very few side effects, that’s a really good investment of our time.”
After discovering the mini-protein, Olson turned to chemists in Fred Hutch’s Molecular Design and Therapeutics core facility. They spent several years developing a way to link it to drugs. The team initially paired their peptide with a steroid called dexamethasone. But they discovered that small amounts of the drug leaked into the rats’ bloodstream, causing the same side effects they aimed to eliminate.
The team ended up using another steroid called Triamcinolone acetonide, or TAA. Their tests in rats showed it was as effective at treating inflammation as dexamethasone. But when TAA gets into the bloodstream, it becomes inactive. So: no detectable side effects.
Emily Girard, staff scientist in Olson’s lab and one of the study’s lead authors, said the work showed what team research can accomplish.
“It’s a pretty simple idea to take a mini-protein that naturally goes to cartilage and attach something to it so that you get targeted delivery of the drug, but it was challenging to accomplish,” Girard said. “We had to learn and adapt the behavior of the mini-protein, the chemical linker and the steroid payload to make a product that would go to cartilage, stay as long as we needed it to, release the drug at the right rate, and have a local but not systemic effect. There is more development to be done, but I hope that this work results in a therapeutic that will help a lot of people.”
The team hopes to test this approach in patients through a clinical trial, Olson said. But first they’ll need to answer several more unknowns with further toxicology and other studies.
Olson added that while this study involved steroids, it shows that these mini-proteins could deliver other drugs into cartilage. “We think that steroids have important potential as a candidate for clinical development and we’re actively exploring other payloads that could be delivered to the joints,” he said. “The long-term goal is to deliver molecules that go beyond controlling arthritis to actually reversing it.”

Leave a Reply