HELMHOLTZ ZENTRUM MÜNCHEN – GERMAN RESEARCH CENTER FOR ENVIRONMENTAL HEALTH

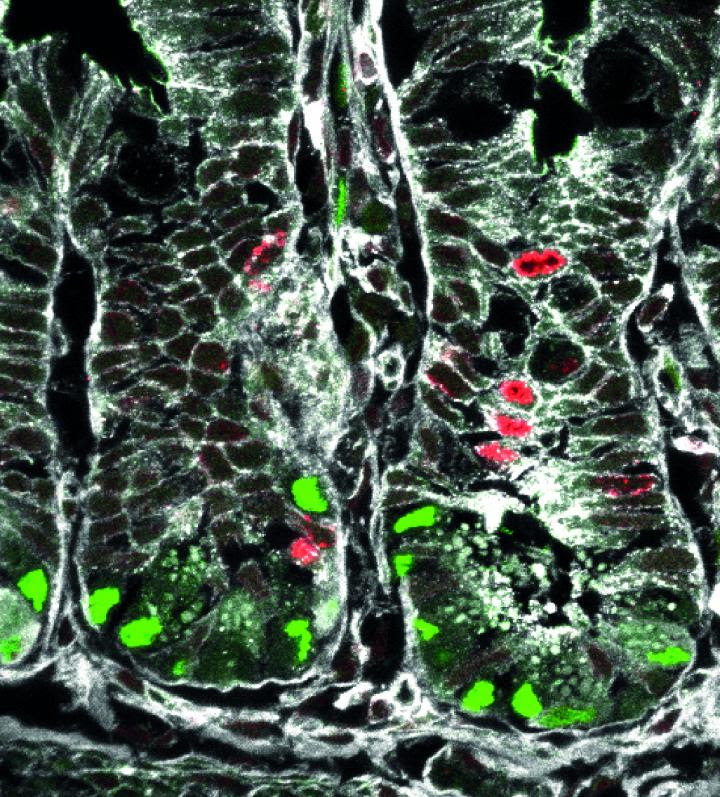
IMAGE: ACTIVATION OF THE WNT/PCP SIGNALING PATHWAY (SHOWN IN GREEN) IN INTESTINAL STEM CELLS PRIMES THEIR FATE TOWARDS THE PANETH AND ENTEROENDOCRINE LINEAGE.
CREDIT: HELMHOLTZ ZENTRUM MÜNCHEN
The gut plays a central role in the regulation of the body’s metabolism and its dysfunction is associated with a variety of diseases, such as obesity, diabetes, colitis and colorectal cancer that affect millions of people worldwide. Targeting endocrine dysfunction at an early stage by stimulating the formation of specific enteroendocrine cells from intestinal stem cells could be a promising regenerative approach for diabetes therapy. For this, however, a detailed understanding of the intestinal stem cell lineage hierarchy and the signals regulating the recruitment of the different intestinal cell types is critical.
Heiko Lickert and his research group have taken up this challenge. Lickert is director of the Institute of Diabetes and Regeneration Research at Helmholtz Zentrum München, professor of beta cell biology at Technical University of Munich (TUM) and member of the German Center for Diabetes Research (DZD). In the following, Lickert and first author Anika Böttcher talk about their latest paper on the basic mechanisms of intestinal stem cell function published in Nature Cell Biology.
Why is the gut so important for health research?
Heiko Lickert: As the body’s digestive and largest endocrine system, the gut is central to the regulation of energy and glucose homeostasis. Intestinal functions are carried out by specialized cells which are constantly generated and renewed every 3-4 days from intestinal stem cells. For example, so-called enteroendocrine cells produce over 20 different types of hormones that signal to the brain and pancreas to regulate for instance appetite, food intake, gastric emptying and insulin secretion from pancreatic beta cells. Another important gut function is exerted by so-called Paneth cells that produce defensins and protect against invading pathogens. Consequently, it is not surprising that intestinal dysfunction is associated with a variety of diseases, such as chronic inflammation,colorectal cancer and diabetes, affecting millions of people worldwide.
What were the most important findings in your latest research about intestinal stem cells?
Anika Böttcher: We improved our understanding of how intestinal stem cells constantly renew and give rise to specialized cell types at unprecedented single cell resolution. Thus, we are now able to describe potential progenitor populations for each intestinal cell and we have shown that for every lineage intestinal stem cells give rise to unipotent lineage progenitors. Furthermore, we identified a specific intestinal stem cell niche signal pathway (called Wnt/planar cell polarity pathway) regulating intestinal stem cell self-renewal and lineage decisions. This is very important, as we know that intestinal stem cells can indefinitely renew and maintain the gut function and tissue barrier. Those are 6 meters of epithelium and more than 100 million of cells generated every day in humans! Moreover, these cells differentiate into every single cell type. The risk of failure in this self-renewal or lineage specification process to result in a chronic disease therefore is quite high.
Using a more technical term, we were able to delineate a detailed intestinal stem cell lineage tree and identified new niche signals. In order to obtain those breaking-through results, we integrated time-resolved lineage labelling of rare intestinal lineages using different reporter mouse lines with genome-wide and targeted single-cell gene expression analysis to dissect intestinal stem cell lineage decisions. Together with Fabian Theis’ team of computational biologists at Helmholtz Munich and TUM, we profiled 60,000 intestinal cells. To analyze this data set, we leveraged newly developed machine learning techniques to automatically identify branching lineages and key contributing factors in the gene expression space. The findings are broadly applicable and are equally important for cancer, inflammation and colitis as well as obesity and diabetes.
How can this new knowledge be translated to therapeutic approaches?
Heiko Lickert: This study challenges current paradigms and we advanced our understanding of intestinal stem cell self-renewal, heterogeneity and lineage recruitment. We can use this basic knowledge to map what happens to intestinal stem cell lineage allocation and differentiation during chronic disease. Insights from this will put us in place to develop specific therapies for these diseases by targeting lineage progenitors for example to regenerate the formation of specific cells that are lost during disease progression or to identify and eradicate intestinal cancer stem cells. Specifically, at our institute, we will focus our efforts on diabetes.

Leave a Reply