by Delthia Ricks, Medical Xpress
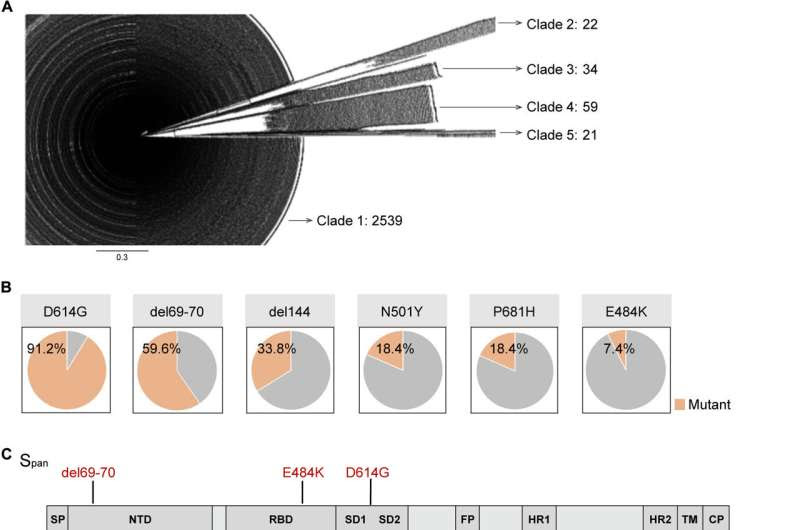
SARS-CoV-2 S protein evolution was used to guide the design of the Span sequence.(A) Phylogenetic trees are shown for 2675 S protein amino acid sequences from the NCBI database. (B) Proportion of the top five mutations and the E484K mutation for the amino acid sequences analyzed. (C) Linear diagram of the full-length SARS-CoV-2 S protein, with Span mutations in red. (D and E) The phylogenetic tree was constructed using the neighbor-joining method by MEGA 10.0 and displayed either in a rectangle shape (D) or in a radiate shape (E). The Span antigen is highlighted in red (the line of Span is magnified 10-fold for visualization). Credit: Science Translational Medicine (2023). DOI: 10.1126/scitranslmed.abo3332
By taking inspiration from the evolutionary history of SARS-CoV-2 itself, scientists in China have crafted a new vaccine that, at least in animal models, provides protection against omicron and an array of its subvariants.
Even though early generations of SARS-CoV-2 vaccines and boosters have been extremely successful, viral evolution and the emergence of immune evasion have made subsequent vaccines more difficult to produce. Vaccinologists are constantly asking: How do we keep up with the rapidly evolving pathogen? The coronavirus always seems several steps ahead, especially now as omicron subvariants continue to evolve keener capacities of immune escape.
Despite the invaluable tools that mRNA vaccines have become over the past two years, more vaccine approaches are needed, scientists say.
As it turns out, the viral spike protein has regions that have remained highly conserved across recent SARS-CoV-2 variants. These conserved regions are veritable catalogs of evolutionary data with which to craft a next-generation vaccine. The spike protein is the business end of the virus that binds to human ACE-2 receptors to initiate infection.
To address the critical problem of immune escape and to begin the arduous task of developing a new vaccine, Dr. Yongliang Zhao and colleagues at State Key Laboratory of Virology, a division of Wuhan University in China, hope to blunt the impact of subvariants in the future with a new kind of vaccine.
The experimental vaccine they are already testing in lab mice is based on conserved regions of the spike protein, which means the vaccine is intimately linked to parts of the spike that rarely mutate.
The Wuhan team is quietly voicing optimism about their research, which they hope will serve as a model for future vaccines, a pan-protective immunization—a universal shot that guards against existing variants and threats that may arise in the future. A key concern with current vaccines is that while they protect against severe disease, newer versions of the virus, such as omicron’s growing multitude of subvariants, can slip past the immune system’s defenses.
Zhao and a team of collaborators from several Wuhan research institutions began development of the new vaccine by interrogating the evolutionary trajectory of SARS-CoV-2. As a critical part of their research, they analyzed more than 11 million SARS-CoV-2 sequences, as well as the infectivity and immune escape ability of 54 SARS-CoV-2 pseudoviruses and variants.
The experiments revealed that the viral spike protein hasn’t evolved randomly but rather towards either high infectivity paired with low immune escape or low infectivity paired with high immune escape.
Based on this, the team designed a vaccine centered on a newly engineered antigen named Span. The letter “S” in the name stands for “spike,” as in the viral spike protein, and “pan” is from the Greek word meaning “all,” referring to a vaccine against all spike proteins. Looking at the term pan another way, combining it with a version of the Greek word demos, which means people, you get “pandemic,” a disease that affects all people.
Developing the investigational Span vaccine hasn’t been easy, Zhao and colleagues write in the journal Science Translational Medicine. “SARS-CoV-2 continues to accumulate mutations to evade immunity, leading to breakthrough infections after vaccination,” asserted Zhao, lead author of the Span vaccine research.
“How researchers anticipate the evolutionary trajectory of the virus in advance in the design of next-generation vaccines requires investigation,” Zhao continued. “Here, we performed a comprehensive study of 11,650,487 SARS-CoV-2 sequences, which revealed that the SARS-CoV-2 spike protein evolved not randomly but into directional paths of either high infectivity plus low immune resistance or low infectivity plus high immune resistance.”
The Span antigen incorporates amino acid residues found at a high frequency across all major variants to date, allowing it to protect against evolutionarily divergent lineages. The Span vaccine so far has prompted neutralizing antibodies against various SARS-CoV-2 variants when the vaccine is administered to animal models in the laboratory.
Zhao and collaborators tested the innovative Span vaccine alongside a vaccine made from a wildtype spike protein that lacked the evolutionary dowry of Span. The researchers found that the immune response was more potent after Span vaccination than shots based on the wildtype spike protein. Animal models—in this case, mice—were completely protected from omicron variants when administered shots of the Span vaccine.
The Wuhan team, according to Zhao, needs to conduct additional tests of the vaccine’s effectiveness against more recent omicron subvariants, particularly those that have emerged in recent months. The researchers also caution that Span inoculations require more testing in animal models other than lab mice to best understand the vaccine’s strengths and weaknesses. “It will be essential to include additional animal models before translating this vaccine to [human] clinical trials,” Zhao concluded.

Leave a Reply