by Walter and Eliza Hall Institute of Medical Research
Credit: Molecular Cell (2023). DOI: 10.1016/j.molcel.2023.04.021
While mitochondria play a crucial role in producing the energy our cells need to carry out their various functions, when damaged, they can have profound effects on cellular function and contribute to the development of various diseases.
Broken-down mitochondria are usually removed and recycled through a garbage disposal process known as “mitophagy.”
PINK1 and Parkin are two proteins vital to this process, responsible for “tagging” malfunctioning mitochondria for destruction. In Parkinson’s disease, mutations in these proteins can result in the accumulation of damaged mitochondria in the brain, which can lead to motor symptoms such as tremors, stiffness and difficulty with movement.
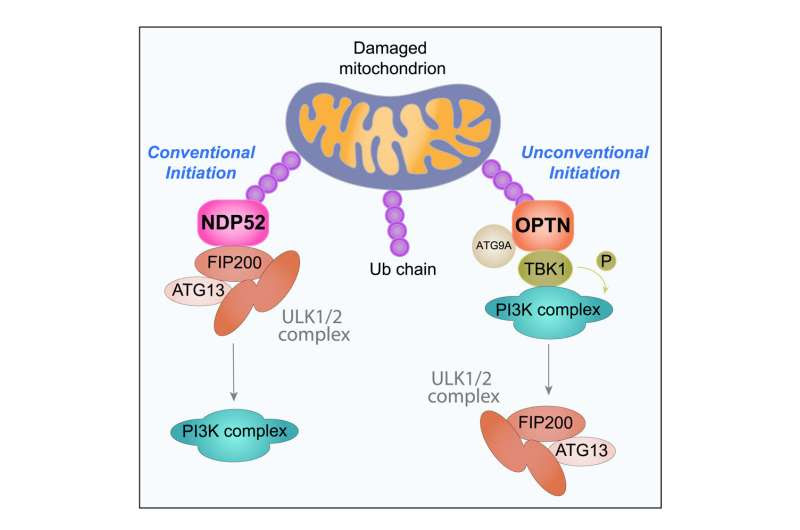
The new research, published in Molecular Cell, solves a mystery about how the protein Optineurin recognizes unhealthy mitochondria tagged by PINK1 and Parkin, enabling their delivery to our body’s garbage disposal system.
Associate Professor Michael Lazarou, a Laboratory Head in WEHI’s Ubiquitin Signaling Division, said the discovery filled a vital knowledge gap that would transform our understanding of this cellular pathway.
“Until this study, Optineurin’s precise role in initiating our body’s garbage disposal process was unknown,” Assoc Prof Lazarou, who also holds a co-appointment with Monash University, said.
“While there are many proteins that link damaged cellular materials to the garbage disposal machinery, we found that Optineurin does this in a highly unconventional way that is unlike anything else we’ve seen from similar proteins. This finding is significant because the human brain relies on Optineurin to degrade its mitochondria through the garbage disposal system driven by PINK1 and Parkin.
“Knowing how Optineurin does this provides us with a framework on how we might be able to target PINK1 and Parkin mitophagy in disease and prevent the build-up of damaged mitochondria in neurons as we age. Achieving this would be instrumental to people with Parkinson’s disease—a condition that continues to impact more than 10 million people worldwide, including 80,000 Australians.”
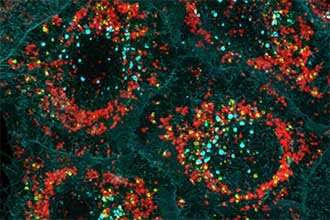
Cellular “garbage bags” being built through Optineurin (green), around damaged mitochondria (red). Credit: WEHI
Outlier discovery
PINK1 acts as a “watch-house” inside the mitochondria, responsible for monitoring their health. When it detects problems, it activates Parkin, which tags damaged mitochondria for removal. They work together to instruct our body to generate cellular “garbage bags” around broken-down mitochondria and enlist the help of Optineurin to initiate this process.
The new study revealed that Optineurin removes damaged mitochondria by binding to an enzyme known as TBK1. From there, they found that TBK1 goes on to activate a specific cellular machine that is key to generating these garbage bags around unhealthy mitochondria.
First author Dr. Thanh Nguyen said, “Other proteins don’t need TBK1 to help them trigger this degradation process, making Optineurin a real outlier when it comes to how our bodies remove mitochondria. This has allowed us to look at the features of this pathway involving TBK1 as a potential drug target, which is a significant step forward in our search for new Parkinson’s disease treatments.
“The ultimate goal would be to find a way to boost levels of PINK1/Parkin mitophagy in the body—especially the brain—so that damaged mitochondria can be removed more effectively. We also hope to design a molecule that could mimic what Optineurin does, so damaged mitochondria could be removed even without PINK1 or Parkin.
“Given Optineurin is critical in activating the garbage disposal system in our brains, this could then prevent the accumulation of damaged mitochondria in this region, which is a significant precursor to Parkinson’s disease.”
Dr. Nguyen said while clinical application of the research is years away, the discovery had laid the essential foundation needed to understand how Optineurin works and to realize the pathway’s potential as a future therapeutic target.
“Our next step is to work with WEHI’s Parkinson’s Disease Center to validate our findings in neuronal model systems to understand why Optineurin behaves this way, which will provide further insight into how we can target Optineurin and TBK1 to enhance treatment options for people with PINK1/Parkin mutations in the future.”

Leave a Reply