by University of Tokyo
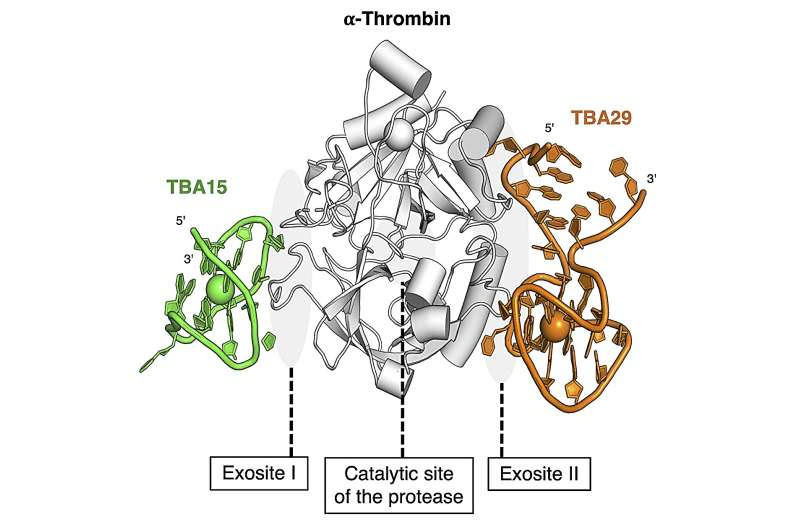
A new DNA drug to fight blood clots—a potential new and less risky treatment for thrombosisHuman thrombin. Thrombin is the enzyme responsible for bleeding and for blood clotting, depending on how it’s activated, and has two DNA drug-binding sites. Credit: Yoshimoto et al, CC-BY
Various medical circumstances, including heart attacks and extreme cases of COVID-19, necessitate the use of anticoagulants, medicines that prevent blood clots. But the most commonly used, heparin, can induce potentially fatal side effects by making the blood clots worse rather than better. This only happens in a minority of patients so effective treatments are not commonly explored.
For the first time, researchers, including those from the University of Tokyo, have proposed a side effect–free anticoagulating treatment that has so far proved effective in test mice and could be ready for human trials in just a few years. The research is published in the journal Molecular Therapy—Nucleic Acids.
Although much of the world seems to have moved on from the COVID-19 pandemic, the effects continue to linger. One aspect of some of the extreme cases of COVID-19 that has not been widely reported is the complication brought on when the anticoagulant medicine heparin is used in an attempt to reduce blood clots. A small number of patients—up to 3% of recipients—suffer the side effect heparin-induced thrombocytopenia (HIT), a potentially fatal and rapid clotting of the blood, the opposite of the intended effect.
Heparin was the first anticoagulant and is used widely—it’s considered incredibly important by the World Health Organization. But, because of the low number of HIT sufferers and thus lack of interest from the pharmaceutical industry, this issue is underexplored, despite the side effect’s severity and increased incidence due to COVID-19. It can be especially problematic in pregnant women as they cannot take existing treatments due to the potentially adverse affects on the fetus.
A new DNA drug to fight blood clots—a potential new and less risky treatment for thrombosisNew DNA drug Pse08-29. The short DNA sequence in purple, [M08s G2]c, is the antidote to prevent severe bleeding in the treatment using the bispecific aptamer Pse08-29 for heparin-induced thrombocytopenia. Credit: Yoshimoto et al. CC-BY
“The best treatment for HIT is an infusion of what are called thrombin inhibitors, but current drugs can lead to severe bleeding and there is no antidote to avoid this,” said Associate Professor Keitaro Yoshimoto from the Department of Life Sciences at the University of Tokyo.
“Ideally, we could avoid HIT altogether. But at present, that is not possible, so we need a new low-risk thrombin inhibitor to replace current drugs. My team and I have created such an anticoagulant and have demonstrated it in mice and also in human blood plasma.”
The team devised a next-generation thrombin inhibitor consisting of DNA molecules which includes a novel mechanism to prevent the severe bleeding. The key molecule in the drug is called a bispecific aptamer and its special feature is being able to bind to multiple things simultaneously. Another useful feature is short DNA sections which act as an antidote to the undesirable clotting side effect during HIT.
This DNA-based drug essentially enables more complex behaviors than drugs based on simpler, more traditional chemistry. From their studies in mice, the team has shown the treatment is around 10 times as effective as the current best treatments for HIT.
An additional benefit to pregnant women is that the nucleic acid-based drug and accompanying antidote do not cross the placenta to the fetus, as the DNA molecules in the drug are too large to cross the barrier presented by the placenta.
This research came about as Yoshimoto has a history in biochemistry and the science of molecular separation, specializing in a method called MACE-SELEX for the selection of aptamers, short sections of DNA useful for medicine. He teamed up with Assistant Professor Asuka Sakata of Nara Medical University in Japan who specializes in thrombosis biology, and together with their team, they embarked on using Yoshimoto’s ideas to solve medical issues raised in Sakata’s research.
“We hope to proceed with human trials soon,” said Yoshimoto. “It will take up to two years for preclinical studies and five years to complete clinical trials in humans. Though the number of HIT sufferers is small, it’s such a severe condition I feel it’s important we tackle it quickly.”
More information: Masanobu Nagano et al, A neutralizable dimeric anti-thrombin aptamer with potent anticoagulant activity in mice, Molecular Therapy—Nucleic Acids (2023). DOI: 10.1016/j.omtn.2023.07.038
Provided by University of Tokyo

Leave a Reply