Peer-Reviewed Publication
FIRST HOSPITAL OF JILIN UNIVERSITY
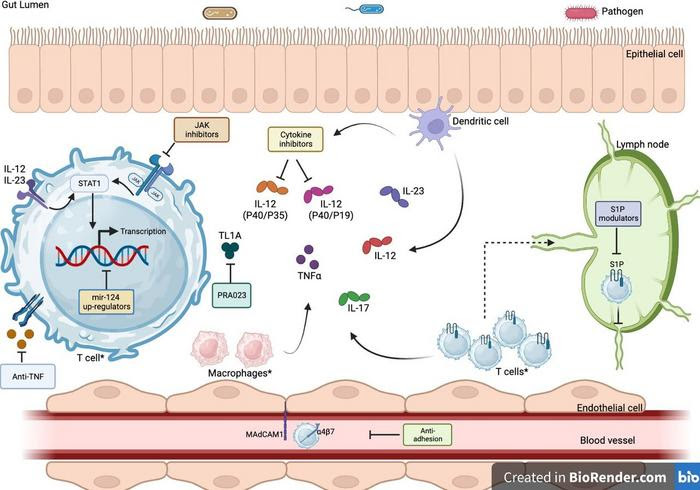
ANTITUMOUR NECROSIS FACTOR ALPHA (ANTI-TNFΑ) AGENTS INCLUDE INFLIXIMAB, ADALIMUMAB, GOLIMUMAB, AVX-470 AND OPRX-106. JANUS KINASE (JAK) INHIBITORS: TOFACITINIB, FILGOTINIB AND UPADACITINIB. CYTOKINE INHIBITORS: USTEKINUMAB, WHICH TARGETS INTERLEUKIN (IL) 12, SUBUNIT P40/P35; RISANKIZUMAB, MIRIKIZUMAB, GUSELKUMAB AND BRAZIKUMAB TARGET IL-23, SUBUNIT P40/P19. SPHINGOSINE-1-PHOSPHATE (S1P) RECEPTOR MODULATORS: AMISELIMOD, OZANIMOD, ETRASIMOD AND FINGOLIMOD. ANTIADHESION AGENTS: VEDOLIZUMAB, ETROLIZUMAB AND ONTAMALIMAB. THE MICRORNA-124 AGENT IS OBEFAZIMOD. * INDICATES THE CELLS WHICH PHOSPHODIESTERASE (PDE) INHIBITORS SUCH AS APREMILAST ACT UPON. IMAGE CREATED THROUGH THE USE OF www.biorender.com. MADCAM-1, MUCOSAL ADDRESSIN CELL ADHESION MOLECULE 1; STAT, SIGNAL TRANSDUCER AND ACTIVATOR OF TRANSCRIPTION; T CELL, T LYMPHOCYTES; TL1A, TUMOUR-NECROSIS FACTOR-LIKE CYTOKINE 1A; Α4Β7, ALPHA 4 BETA 7.
CREDIT: BY DR ADITI KUMAR AND DR PHILIP J SMITH
Ulcerative colitis (UC) and Crohn’s disease (CD) are chronic inflammatory bowel diseases (IBD) that affect the gastrointestinal tract. In recent decades, there have been significant advances in the understanding of IBD pathophysiology and the development of new treatments.
The International Organisation for the Study of Inflammatory Bowel Diseases (IOIBD) developed the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) programs, which recommend specific treatment goals for UC and CD in children and adults. These goals include clinical response and remission, endoscopic healing, normalization of C-reactive protein/erythrocyte sedimentation rate and fecal calprotectin, prevention of disability, restoration of quality of life, and normal growth in children.
The current mainstay treatments for IBD include immunomodulators, biologics (anti-tumor necrosis factor alpha (TNF-α) agents being the most commonly used), and other monoclonal antibodies such as anti-integrins and anti-interleukins (IL-12/23). However, primary and secondary loss of response to these therapies is a major recurring issue, with often diminishing returns in terms of efficacy for the next line of therapies prescribed.
Recently, there has been an influx of new and emerging medications entering the market that are showing promising efficacy results in patients with moderate-to-severe IBD who have previously failed to respond to multiple drugs. These novel and emerging therapies include:
- Subcutaneous infliximab and oral anti-TNFα preparations: These drugs offer greater convenience and flexibility for patients compared to traditional intravenous anti-TNFα agents.
- Antiadhesion agents: These drugs block the interaction between leukocytes and the intestinal lining, preventing inflammation.
- Cytokine inhibitors: These drugs target specific cytokines that play a role in IBD inflammation, such as IL-12/23 and IL-17.
- Janus kinase (JAK) inhibitors: JAKs are signalling proteins involved in a variety of inflammatory pathways. JAK inhibitors have shown promise in the treatment of IBD, but more research is needed.
- Phosphodiesterase (PDE) inhibitors: PDEs are enzymes that break down cyclic AMP (cAMP) and cyclic GMP (cGMP), two signalling molecules that play a role in inflammation. PDE inhibitors have been shown to be effective in treating IBD in animal models, and clinical trials are underway.
- Sphingosine-1-phosphate receptor (S1PR) modulators: S1PRs are involved in the trafficking of leukocytes to the intestinal lining. S1PR modulators have shown promise in the treatment of IBD in clinical trials.
- MicroRNA-124 (miR-124) upregulators: miR-124 is a microRNA that has anti-inflammatory properties. miR-124 upregulators are being developed to treat IBD, but more research is needed.
These novel and emerging therapies offer new hope for patients with moderate-to-severe IBD who have previously failed to respond to multiple drugs. More research is needed to confirm their long-term safety and efficacy, but they have the potential to revolutionize the treatment of IBD.
See the article:
Kumar A, Smith PJ. Horizon scanning: new and future therapies in the management of inflammatory bowel disease.
eGastroenterology 2023;1:e100012. doi: 10.1136/egastro-2023-100012
About the eGastroenterology
eGastroenterology is a new, open-access, and open peer-reviewed BMJ Journal, which focuses on basic, clinical, translational, and evidence-based medicine research in all areas of gastroenterology (including hepatology, pancreatology, esophagology, and gastrointestinal surgery).
For more information, please visit: egastroenterology.bmj.com and follow us on Twitter (@eGastro_BMJ).
Sign-up to Email Alerts for eGastroenterology: https://emails.bmj.com/k/Bmj/jausu/egastroenterology
JOURNAL
eGastroenterology
DOI
10.1136/egastro-2023-100012
ARTICLE TITLE
Horizon scanning: new and future therapies in the management of inflammatory bowel disease

Leave a Reply