by University of Birmingham
Credit: Cell Reports (2023). DOI: 10.1016/j.celrep.2023.113568
A highly aggressive common form of leukemia which is activated by mutations in signaling molecules is maintained by a web of regulatory proteins downstream of these signals.
New research published in Cell Reports shows that a complex network of interacting genes activated by this altered signaling may be able to be manipulated to kill off Acute Myeloid Leukemia (AML) cancer cells.
This study by an international team of researchers from the University of Birmingham, Newcastle University, the Princess Maxima Center of Pediatric Oncology in Utrecht and the University of Virginia, U.S. has used advanced screening tools to identify how gene regulatory networks (GRNs) maintain a sub-type of blood cancer called FLT3-ITD mutated AML.
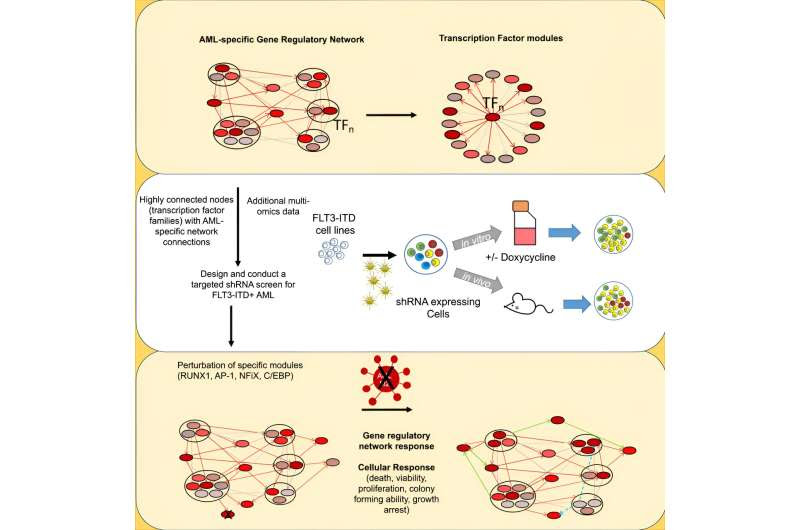
Using this AML model, the researchers have identified connections between proteins called transcription factors (TF) and the genes they bind to that together form a complex network (GRN) that is highly specific for FLT3-ITD AML cells as compared to healthy cells.
Using a screening method developed in Newcastle, the researchers identified about 100 genes within this GRN that are important for AML growth and survival. They homed in on several of these genes to study the effect of targeting them. The TF RUNX1 was studied in fine detail and the analyses showed that RUNX1 is a key factor in keeping the GRN stable.
Significantly, the RUNX1 protein could be blocked by using a small molecule inhibitor developed by Prof John Bushweller of the University of Virginia, leading to the collapse of the network that maintains FLT3-ITD AML.
Professor Constanze Bonifer from the Institute of Cancer and Genomic Sciences at the University of Birmingham and senior author of the paper said, “The FLT3-ITD sub-type of acute myeloid leukemia that we have been studying has very poor outcomes with high relapse rates among those who do go into remission. We set out to identify very specific targets that are required for AML cancer cells to regulate themselves, that could potentially lead to new treatments.
“We are delighted to have identified multiple factors, including TFs and signaling proteins that have key roles in maintaining these gene regulation networks where TFs and genes are wired in an AML-specific way. Such networks act a bit like a computer program that runs processes to maintain AMLs and which are different to those networks found in normal cells. Our research has found that knocking out such factors resulted in the network shutting down, and which may lead to the cancer cells dying off as they are unable to replicate.”
Modeling AML
To identify potential protein targets that regulate AML cancer cells, the researchers mapped the transcription factors and their genes. The team used a combination of normal and malignant cells to then work out which gene expressions were promoting AML survival.
The team then used a type of screening technique called shRNA to look at specific transcription factors’ role in maintaining the network for AML. As the TFs are clustered together in an interactive network, the team then observed the effect of targeting individual factors on the whole network, and found that specific proteins including RUNX1 were crucial for the maintenance of the GRN as a whole.
Professor Olaf Heidenreich who is a co-grant holder on this project with his colleague Dr. Helen Blair from Newcastle and who is now at the Princess Maxima Center for Pediatric Oncology in Utrecht said, “Many researchers in the world use techniques called ‘genome wide screening’ where they eliminate every gene in cancer cells to identify those genes that are essential for the growth of these cells.
“However, this method identifies many genes that are also required for healthy cells. Therefore, finding genes that are only important for cancer cells is a bit like finding a needle in a haystack. Identifying the gene regulatory networks that are specific for cancer cells makes this talks much easier.
“Besides testing the effect of select targets on AML growth, our work will provide an important resource for the scientific community to home in on the targets that really matter.”
More information: Daniel J.L. Coleman et al, Gene regulatory network analysis predicts cooperating transcription factor regulons required for FLT3-ITD+ AML growth, Cell Reports (2023). DOI: 10.1016/j.celrep.2023.113568
Journal information: Cell Reports
Provided by University of Birmingham

Leave a Reply