by Jim Oldfield, University of Toronto
Graphical abstract. Credit: Cell Reports Medicine (2023). DOI: 10.1016/j.xcrm.2023.101051
Researchers at the University of Toronto and its partner hospitals are finding that changes in gut microbiota after bariatric surgery can directly improve metabolism, independent of food intake, weight loss and other metabolic factors.
Their ongoing work—including a study published in the journal Cell Reports Medicine focused on patients who underwent the surgery—suggests that microbiome-based therapies such as probiotics and fecal matter transplants have the potential to improve metabolic health.
They may also one day reduce the need for weight-loss surgery itself.
“We know the microbiome contributes to metabolic improvements after bariatric surgery, but have known very little about how,” said Johane Allard, a clinician-scientist at University Health Network and a professor at U of T’s Temerty Faculty of Medicine. “We’ve recently shown that with no other changes, the altered microbiome influences that outcome, and we identify potential mechanisms.”
Bariatric surgery is a cornerstone of treatment for severe obesity. It changes the size and structure of the digestive system, limiting the amount patients can eat and absorption of nutrients. But it also comes with short- and long-term health risks, and in Canada often costs the health-care system more than $20,000 per procedure.
The surgery also releases gut hormones that improve insulin sensitivity and reduce appetite, and it alters the makeup and function of gut microbiota—changes that have surprised and perplexed clinicians, as the annual number of procedures worldwide has surged past half a million.
Researchers have sought to understand how—and how much—these additional biochemical changes contribute to metabolic improvements and weight loss, with an eye to new treatments. But studies of microbiota-related changes that could be harnessed as broadly effective therapies have been stymied by vast differences in gut microbiota among individuals.
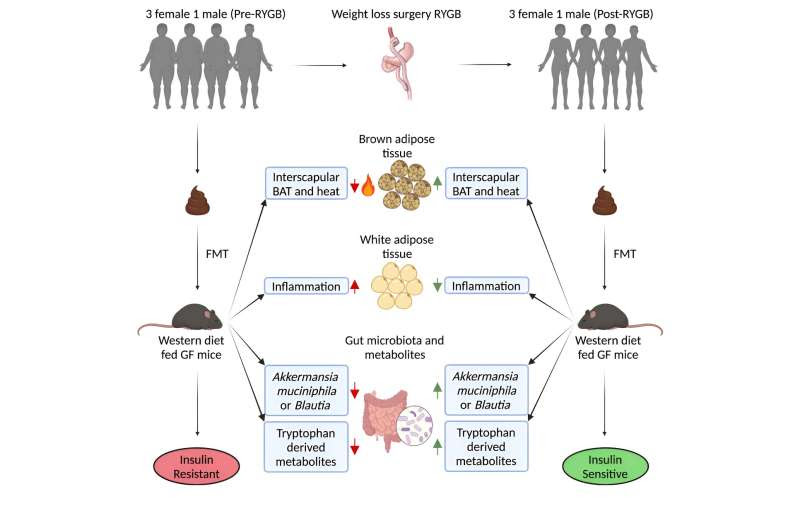
The recent pre-clinical study gets around that problem through paired fecal matter transplants. The Toronto team transferred fecal matter from four people to mice—both before and after the human participants had bariatric surgery. The team fed both groups of mice the same high-fat, western diet in a germ-free facility, then observed the effects over several weeks.
Mice receiving the post-surgery transplant showed much better blood-sugar control and insulin sensitivity than the pre-surgery recipients, suggesting a strong role for the microbiome in improved metabolism, despite no change in body weight. The human study participants also became more sensitive to insulin and lost weight, as expected.
Less expected, however, was an increase in mass and energy expenditure in brown fat among mice that received the post-surgery transplant.
“We were very surprised by the data in brown adipose tissue,” said Dana Philpott, a co-principal investigator in the study and professor of immunology at Temerty Medicine. “We thought if we looked at regular [white fat] adipose tissue, we might see decreased fat or an ability to metabolize better, but the finding was very specific to brown fat.”
Heat was an early clue that something of interest was happening in brown fat, which plays a role in regulating body temperature.
“When we first put the post-surgery transplant mice in the metabolic cages, we noticed they generated more heat,” said Jitender Yadav, a postdoctoral researcher in the Philpott lab and a co-first author on the study. “We also noticed in some of the literature that bariatric surgery in mice increases brown adipose tissue and energy expenditure, and in our study, we were able to see the similar effect just by transferring the post-surgery microbiome.”
As well, the team found biochemical and transcriptional markers of reduced inflammation in the white fat of post-surgery mice—another sign of improved metabolic health. To look for microbiota-related changes that could explain all these metabolic improvements, the researchers studied and compared metabolites in stool samples from pre- and post-surgery mice.
They found increases in tryptophan metabolites, short-chain fatty acids and acylcarnitines, and decreases in amino acids, organic acid and lactic acid—all correlated with improved metabolic health. Those changes in metabolites were consistent across the post-surgery mice, despite variations in the constituents of their microbiota and in the microbiota of the four patients.
“The bacterial makeup of the microbiota wasn’t significant,” Yadav said. “We now think that microbiome-based therapies that induce the right mix of metabolites, such as pre- and probiotics, dietary changes and fecal matter transplants, could be an effective therapy for improved metabolism and weight loss.”
Co-principal investigator Herbert Gaisano, a clinician-scientist at Toronto General Hospital Research Institute and a professor in Temerty Medicine’s department of medicine, aims to study how these metabolites work. Gaisano and his team, including co-first authors on the current study Tao Liang and Tairan Qin, will analyze the metabolites in human tissue samples of fat and liver, obtained during bariatric surgery.
The Philpott lab also continues to study the metabolites in the offspring of the study mice, and they hope to replicate their results with a larger number of patients at some point.
More broadly, Yadav said the study should help nudge the field of microbiome research away from a long-standing focus on bacteria. “One takeaway is that the amount and type of bacteria don’t always matter,” he said. “It’s the metabolites they produce, and which get absorbed downstream, that can influence health.”
More information: Jitender Yadav et al, Gut microbiome modified by bariatric surgery improves insulin sensitivity and correlates with increased brown fat activity and energy expenditure, Cell Reports Medicine (2023). DOI: 10.1016/j.xcrm.2023.101051
Journal information: Cell Reports Medicine
Provided by University of Toronto

Leave a Reply