by Nottingham Trent University
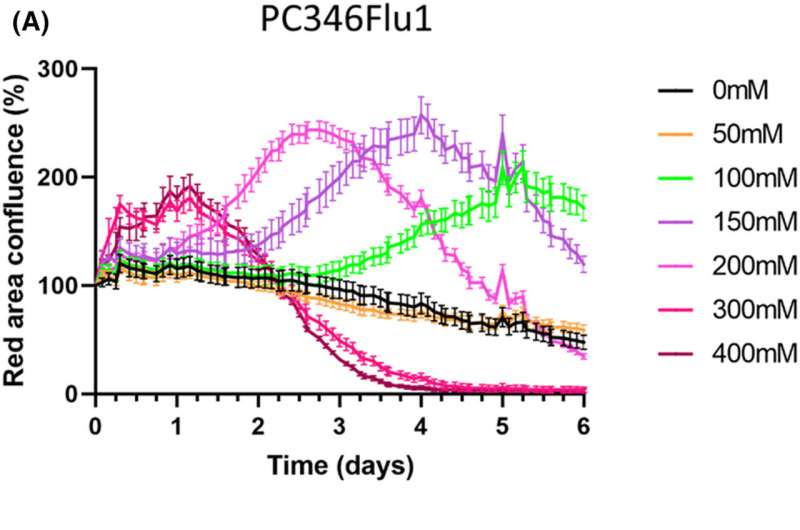
Live-cell cytotoxicity analysis shows that carnosine induces the death of PC346Flu1 (A) and TRAMP-C1 (B) cells. Carnosine inhibited cell growth in a dose- and time-dependent manner due to the relatively different growth rates of the cells studied. The higher the dose of carnosine, the shorter the time required to reach cell death over 6 days for PC346Flu1 cells and over 48 h for TRAMP-C1 cells. The maximum effect was shown starting from carnosine concentration of 300 mM for PC346Flu1 cells and 200 mM for TRAMP-C1 cells. Credit: Journal of Cellular and Molecular Medicine (2023). DOI: 10.1111/jcmm.18061
Scientists at Nottingham Trent University wanted to investigate the anti-cancer properties of carnosine against cells derived from both primary and metastatic prostate cancer—where the cancer began and where it had spread to another part of the body.
Carnosine, which can be produced by the body and is also found in meat, has long been advocated for use as an antioxidant to facilitate healthy aging.
There have been reports of carnosine being effective against the development of a number of different cancers but this is the first time it has been studied in relation to prostate cancer.
The researchers found that carnosine stopped the cells from multiplying and at higher doses even killed cancer from both primary and metastatic cancer cells, while remaining safe to the healthy non-dividing cells.
The research, which also involved University Hospitals Leicester NHS Trust and Manchester Metropolitan University, is published in the Journal of Cellular and Molecular Medicine.
Although carnosine is rapidly degraded by enzymes in the body the researchers argue that it could potentially be an initial treatment for prostate cancer if a constant slow release mechanism is used.
This could include injecting carnosine inside the tumor and releasing it in sufficient quantity before it begins to degrade and lose its effect.
Another approach could be through administration of carnosine-like molecules that are resistant to enzymatic degradation.
The hope then would be that the tumor growth could be monitored via the level of prostate specific antigen (PSA) in the blood and if it continued to grow then the patient would have the option for surgery.
If surgery is performed first it can lead to detrimental scarring as tissues fuse together potentially complicating further surgery.
Prostate cancer is the most common cancer in men in the U.K., with more than 52,000 diagnosed every year on average. One in eight men will be diagnosed with prostate cancer in their lifetime.
Current treatments for organ-confined prostate cancer are not cancer-specific and are commonly accompanied by side effects including urinary incontinence and erectile dysfunction.
Treatments include radiation therapy, chemotherapy, hormone deprivation therapy or a prostatectomy—the partial or complete removal of the prostate through surgery.
“Our results show that carnosine has a significant inhibitory effect in vitro on the proliferation of human prostate cancer cell lines,” said lead researcher Dr. Stephanie McArdle, a scientist in Nottingham Trent University’s John van Geest Cancer Research Center.
“While carnosine has previously been shown to have an anti-tumor effect, its potential role in prostate cancer cells specifically was unknown.
“These are encouraging results and support the need for further human in vivo work to determine the potential use of carnosine as a first line of treatment against the disease.
“It is possible that carnosine-based strategies could be used alone or as a supplementary therapy to surgical or other conventional treatments.”
More information: K. Habra et al, Anticancer actions of carnosine in cellular models of prostate cancer, Journal of Cellular and Molecular Medicine (2023). DOI: 10.1111/jcmm.18061
Provided by Nottingham Trent University

Leave a Reply