by UT Southwestern Medical Center
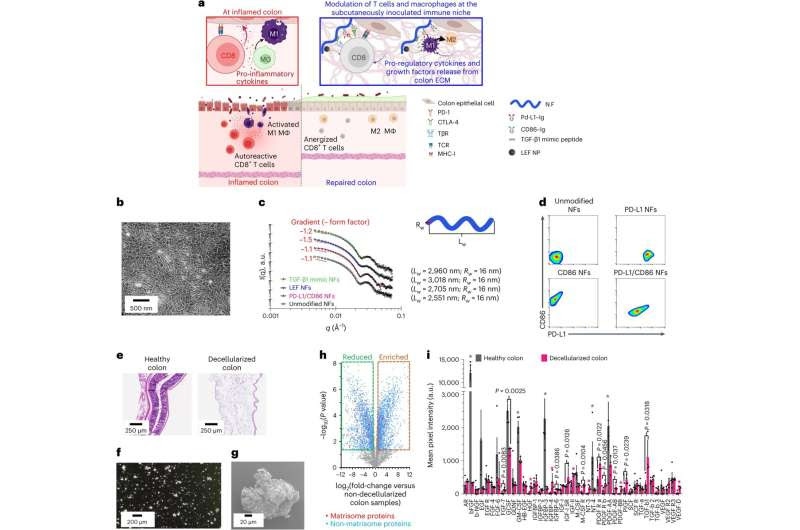
Bioengineering and characterization of the immunosuppressive combinational colon-specific immune niche. Credit: Nature Biomedical Engineering (2023). DOI: 10.1038/s41551-023-01136-9
By taking advantage of mechanisms that allow cancer cells to evade immune attack, UT Southwestern Medical Center researchers have developed a new strategy in animal models that has potential for treating ulcerative colitis. Their findings, reported in Nature Biomedical Engineering, could eventually provide relief to millions of people worldwide who have this or other autoimmune conditions.
“We’re borrowing something that cancer uses for evil and making it into something good,” said senior author Andrew Wang, M.D., Professor and Vice Chair of Translational Research and Commercialization in the Department of Radiation Oncology and a member of the Harold C. Simmons Comprehensive Cancer Center at UT Southwestern. Dr. Wang co-led the study with first author Kin Man Au, Ph.D., Assistant Professor of Radiation Oncology.
For decades, Dr. Wang explained, researchers have known that the immune system can recognize and kill cancers, keeping most malignancies in check. However, cancers can develop the ability to escape the immune system, producing proteins in their microenvironment that suppress immune cell activity and allow tumors to flourish. Conversely, autoimmune conditions develop when the immune system mistakes healthy cells as foreign invaders and launches unnecessary immune attacks.
Dr. Wang and his colleagues realized that they could take a page from cancer’s playbook, retraining the immune system to suppress its activity against specific cell types attacked in autoimmune diseases. Previous studies have used this approach in animal models of type 1 diabetes and multiple sclerosis.
This latest study focuses on ulcerative colitis, a chronic disease characterized by an autoimmune attack against colon cells. For this and other autoimmune diseases, there is no cure. These conditions are typically treated with systemic immunosuppressors, which can reduce inappropriate immune activity. But they have long-term health complications, including an increased risk of infections and cancer.
The researchers worked with a well-established mouse model of ulcerative colitis that mimics the heavy intestinal inflammation and damage suffered by human patients. Drs. Wang and Au and their colleagues injected the animals with a mixture of colon cells and the extracellular matrix that typically surrounds them—simulating the tissue typically attacked in ulcerative colitis—along with polymer nanofibers chemically altered to carry a variety of proteins and other molecules that cancer cells use to suppress immune activity.
These injections not only significantly reduced symptoms of ulcerative colitis, such as diarrhea, rectal bleeding, weight loss, and inflammation-associated colon shortening, but tissue analysis showed that this treatment also reduced immune cell infiltration into the colon’s lining and its concentration of inflammatory molecules. Within seven days after injection, researchers saw that the colon’s lining appeared completely healed in mice that received the combination. Those treated with only parts of the combination or no injection at all still had actively inflamed colon lesions, the study showed.
The treatment also reduced the number of cancerous colon tumors that were developed by 60% (both animal models and human patients with ulcerative colitis have an increased risk of colon cancer). Additionally, the injections appeared to target only immune activity against the colon and did not suppress immunity broadly in the body. When the researchers gave injections to mouse models of ulcerative colitis that also carried melanoma and colon tumors, these animals responded to immunotherapy for their cancer, which would not be possible if they were systemically immunosuppressed.
Together, Dr. Wang said, these findings suggest that the combination injections could be a viable new way of treating ulcerative colitis. A similar approach may also be used to treat other autoimmune diseases. He and his colleagues have filed a patent to develop this strategy into a clinical treatment.
More information: Kin Man Au et al, An injectable subcutaneous colon-specific immune niche for the treatment of ulcerative colitis, Nature Biomedical Engineering (2023). DOI: 10.1038/s41551-023-01136-9
Journal information: Nature Biomedical Engineering
Provided by UT Southwestern Medical Center

Leave a Reply