Scientists investigate how the Cpeb4 protein mediates the production of osteoclasts, which are involved in bone and joint diseases, including osteoporosis.Peer-Reviewed Publication
TOKYO UNIVERSITY OF SCIENCE
A RECENT STUDY BY TUS RESEARCHERS REVEAL THAT THE CPEB4 PROTEIN MAY HAVE A ROLE IN REGULATING OSTEOCLAST DIFFERENTIATION. THIS, IN TURN, COULD LEAD TO THE DEVELOPMENT OF NEW THERAPEUTIC DRUGS FOR OSTEOPOROSIS AND RHEUMATOID ARTHRITIS.
CREDIT: TADAYOSHI HAYATA FROM TOKYO UNIVERSITY OF SCIENCE
In today’s aging societies, diseases affecting the bones and joints are becoming increasingly common. For example, in Japan alone, over 12 million people suffer from osteoporosis, a condition that severely weakens bones and makes them fragile. If we are to find effective treatments for such disorders, understanding the cellular processes involved in the maintenance of bone and joint tissue is an essential first step.
Osteoclasts are a particularly important type of cell involved in bone maintenance. These cells absorb old or damaged bone and digest it, allowing the body to reuse important materials like calcium and giving way to new bones. As one might expect, various bone diseases arise when osteoclasts do not fulfill their role properly. Scientists have been investigating the mechanisms that regulate the proliferation and differentiation of precursor cells into osteoclasts.
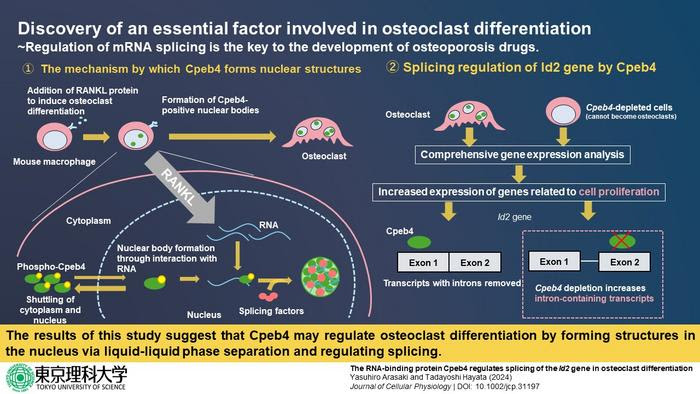
Interestingly, in a study published in 2020, researchers from Tokyo University of Science (TUS) led by Professor Tadayoshi Hayata revealed that the cytoplasmic polyadenylation element-binding protein 4 (Cpeb4) protein is essential in osteoclast differentiation. They also discovered that this protein, which regulates the stability and translation of messenger RNA (mRNA) molecules, transported into specific structures within the nucleus of the cell when osteoclast differentiation was induced. However, just how this relocation occurs and what Cpeb4 exactly does within these nuclear structures still remains a mystery.
Now, in a recent study published in the Journal of Cellular Physiology on 29 January 2024, Prof. Hayata and Mr. Yasuhiro Arasaki from TUS tackled these knowledge gaps. Motivated by the intricate and complex process of osteoclast differentiation, they sought to more thoroughly understand how the “life cycle” of mRNA, i.e., mRNA metabolism, is involved.
First, the researchers introduced strategic modifications into Cpeb4 proteins and performed a series of experiments in cell cultures. They found that the localization of Cpbe4 in the abovementioned nuclear bodies occurred owing to its ability to bind to RNA molecules. Afterwards, seeking to understand the role of Cpeb4 in the nucleus, the researchers demonstrated that Cpeb4 co-localized with certain mRNA splicing factors. These proteins are involved in the process of mRNA splicing, which is a key step in mRNA metabolism. Put simply, it enables a cell to produce diverse mature mRNA molecules (and eventually proteins) from a single gene.
Through RNA sequencing and gene analysis in Cpeb4-depleted cells, they found that Cpeb4 alters the expression of multiple genes associated with splicing events in freshly differentiated osteoclasts. Finally, through further experiments, the researchers revealed that Cpeb4 only altered the splicing patterns of Id2 mRNA, an important protein known to regulate osteoclast differentiation and development.
Overall, this study sheds important light on the mechanisms that regulate osteoclast differentiation. “Through this research, we were able to identify important factors involved in regulating mRNA splicing during the osteoclast differentiation process and obtained new knowledge regarding the control of mRNA splicing during osteoclast differentiation,” comments Prof. Hayata. While the contribution of Cpeb4 is smaller than that of RANKL, a signaling factor that induces osteoclast differentiation, targeting Cpeb4 may have the advantage of reducing the side effects of existing drugs as too much inhibition of osteoclast differentiation with RANKL inhibitory antibodies would halt bone remodeling.
Importantly, the results contribute to a more detailed understanding of how bones are maintained. “Although we used cultured mouse cells in our study, there are also research reports that show a correlation between variations in the CPEB4 gene and bone density in humans,” says Prof. Hayata. “We hope that our findings will help clarify the relationship between these two in the near future.”
Most importantly, the findings of the present study may prove to be a crucial stepping stone for advancing diagnostic techniques and treatments for bone and joint diseases. GWAS analysis has reported a correlation between single nucleotide polymorphisms in introns of the CPEB4 gene region and the estimated bone density. Therefore, it is possible that CPEB4 expression and activity can be used as diagnostic criteria.
However, the researchers note that it is unclear whether Cpeb4 actually regulates bone metabolism in vivo. Therefore, clarification of the molecular basis of Cpeb4 in bone metabolism in mice would help to establish a therapeutic approach. Additionally, recent studies have reported that Cpeb4 is expressed in various cancer cells and contributes to cancer cell survival. In cancer, Cpeb4 contributes to mRNA stability, although splicing regulation may exist.
“The discovery of part of the mechanisms by which Cpeb4 controls osteoclast differentiation could lead to the elucidation of pathologies, including osteoporosis and rheumatoid arthritis, and ultimately become the foundation for the development of new therapeutic drugs,” concludes a hopeful Prof. Hayata.
We too hope these efforts will pave the way for a brighter future for the millions of people suffering from osteoporosis and similar disorders, enabling them to live more active and fulfilling lives.
Reference
DOI: https://doi.org/10.1002/jcp.31197
About The Tokyo University of Science
Tokyo University of Science (TUS) is a well-known and respected university, and the largest science-specialized private research university in Japan, with four campuses in central Tokyo and its suburbs and in Hokkaido. Established in 1881, the university has continually contributed to Japan’s development in science through inculcating the love for science in researchers, technicians, and educators.
With a mission of “Creating science and technology for the harmonious development of nature, human beings, and society,” TUS has undertaken a wide range of research from basic to applied science. TUS has embraced a multidisciplinary approach to research and undertaken intensive study in some of today’s most vital fields. TUS is a meritocracy where the best in science is recognized and nurtured. It is the only private university in Japan that has produced a Nobel Prize winner and the only private university in Asia to produce Nobel Prize winners within the natural sciences field.
Website: https://www.tus.ac.jp/en/mediarelations/
About Professor Tadayoshi Hayata from Tokyo University of Science
Tadayoshi Hayata became an Associate Professor and Principal Investigator at the Department of Molecular Pharmacology, Faculty of Pharmaceutical Science at the Tokyo University of Science in 2018. In 2023, he was promoted to Full Professor. His laboratory focuses on bone metabolism, cellular differentiation, molecular pharmacology, and similar fields to understand the nature of bone and joint diseases and find therapeutic targets. Prof. Hayata is affiliated with several Japanese Societies and the American Society for Bone and Mineral Research. He has published over 60 original articles and given over 150 presentations at academic conferences. In addition, his research on osteoporosis has made it to Japanese newspapers several times.
Funding information
This work was supported by Grant-in-Aid for JSPS Fellows (JP21J22295) and JSPS KAKENHI (JP18K09053).
JOURNAL
Journal of Cellular Physiology
DOI
10.1002/jcp.31197
METHOD OF RESEARCH
Experimental study
SUBJECT OF RESEARCH
Cells
ARTICLE TITLE
The RNA-binding protein Cpeb4 regulates splicing of the Id2 gene in osteoclast differentiation
ARTICLE PUBLICATION DATE
29-Jan-2024
COI STATEMENT
The authors declare that they have no conflicts of interest with the contents of this article

Leave a Reply