By Dr. Chinta SidharthanFeb 27 2024
Reviewed by Susha Cheriyedath, M.Sc.
In a recent study published in the journal Nature Metabolism, a team of scientists investigated whether modulation of the gut microbiome using dietary fiber supplementation in the form of resistant starch could help with insulin resistance and weight loss and offer a potential treatment avenue for metabolic disorders.

Study: Resistant starch intake facilitates weight loss in humans by reshaping the gut microbiota. Image Credit: Sokor Space / ShutterstockStudy: Resistant starch intake facilitates weight loss in humans by reshaping the gut microbiota. Image Credit: Sokor Space / Shutterstock
Background
Obesity has been classified as a global epidemic, with substantial research being conducted on strategies to reduce weight and prevent obesity. It contributes significantly to the global mortality rates by increasing the risk of metabolic diseases such as diabetes, as well as cardiovascular disease risk. Weight management and effective weight loss can lower the risk of these diseases.
Increasing evidence indicates that the gut microbiome plays a pivotal role in the regulation of human physiology and development of various diseases. Gut microbiome composition and diversity are intricately linked to the metabolism of glucose and fat and inflammation.
Furthermore, while fecal microbiome transplantation has been used to establish healthy gut microbiome communities, the procedure has not yielded effective or long-term results. However, diet can be used to modulate the gut microbiome, and dietary interventions, either alone or in conjunction with fecal microbiome transplantation, could potentially improve the clinical outcomes.
About the study
In the present study, the team conducted a randomized, crossover clinical trial involving overweight individuals to determine whether dietary supplementation with resistant starch positively impacted obesity and metabolic phenotypes. They also conducted metagenomic and metabolomic analyses to understand how the resistant starch affected the composition of the gut microbiome and its function.
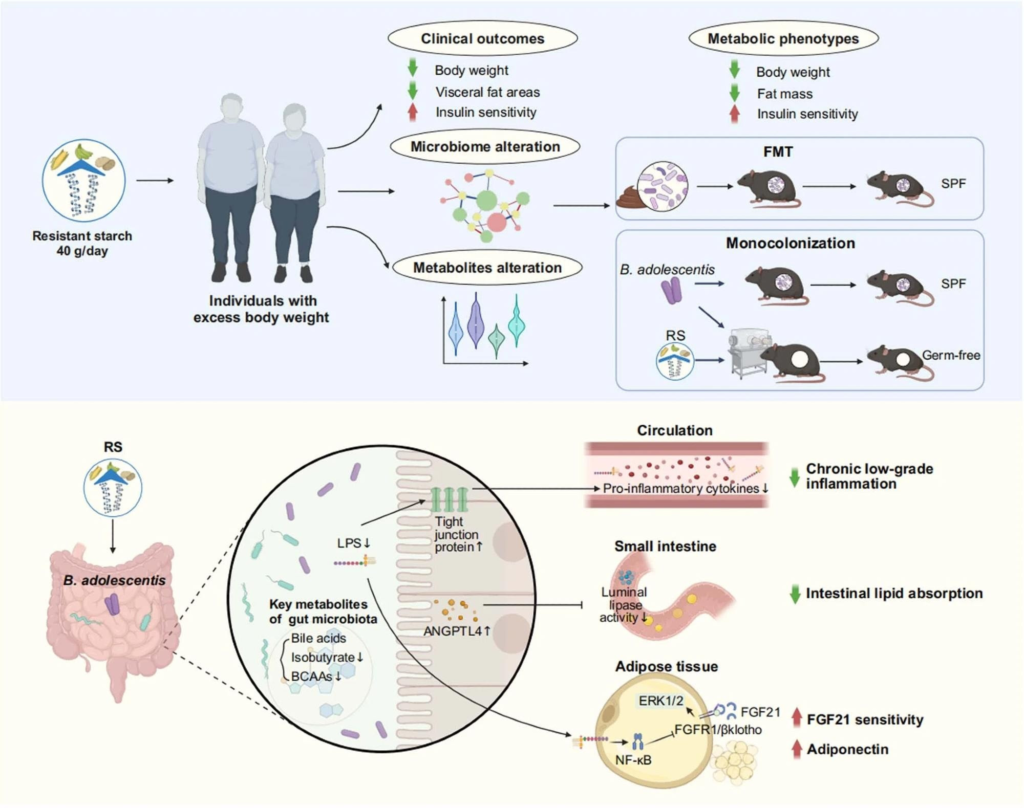
Furthermore, they studied antibiotic-treated mice that had received gut microbiomes from human donors that had already been modified through resistant starch supplementation to understand how gut microbiomes modified through supplementation with resistant starch influence glucose metabolism and adiposity. The metabolomic advantages offered by the gut microbiome modified through resistant starch supplements were also explored.
Resistant starch cannot be broken down by the amylase enzymes produced in humans, functioning as a dietary fiber. During digestion, resistant starch does not get broken down in the stomach or small intestine but moves into the large intestine or colon, where the gut microbiome ferments this dietary fiber. Rodent model studies have shown a decrease in body fat and better metabolic outcomes when the carbohydrate portion of their diet consists mainly of resistant starch.
The present clinical trial included participants with excess body weight who did not have any chronic disorders, were not using any probiotics or antibiotics, and were not undergoing any treatments that would impact their glucose metabolism. The participants were randomly assigned to the treatment or control group, with the treatment group receiving resistant starch in the form of high-amylose maize and the control group receiving amylopectin with no resistant starch.
The starch was provided in sachets in powdered form, and all the participants in the treatment and control groups consumed one packet of the appropriate starch twice a day before a balanced, isoenergetic meal that was provided thrice a day. Since this was a crossover clinical trial, all the participants underwent two eight-week-long interventions, one for the resistant starch treatment and the other for the control treatment.
Results
The results showed that supplementation with resistant starch helped achieve a mean weight loss of about 2.8 kg and improved insulin resistance in overweight participants. The study also found that the beneficial effects of resistant starch supplementation were associated largely with gut microbiome composition changes.
The bacterium Bifidobacterium adolescentis was found to be associated with resistant starch supplementation in humans, and the monocolonization of mice with this bacterium protected them from diet-induced obesity. Resistance starch impacted lipid and fat metabolism by reducing inflammation, restoring the intestinal barrier, and altering the bile acid profile.
The gut microbiota impacts the host physiology through signaling metabolites, of which bile acids play a significant role. Secondary bile acids, such as glycodesoxycholic acid, deoxycholic acid, glycocholic acid, and taurodeoxycholic acid, are important in improving insulin sensitivity and ameliorating hepatic steatosis. The enzyme bile salt hydrolase carries out the deconjugation of secondary bile acids.
The study found that resistant starch supplementation decreased the production of bile salt hydrolase and increased the levels of secondary bile acids. The results were reciprocated in the mice after they were monocolonized with B. adolescentis from humans who underwent resistant starch supplementation.
Resistant starch (RS, 40 g d-1) accompanied with isoenergetic and balanced diets led to an obvious reduction in body weight and improvement of insulin sensitivity, as well as alteration in metagenomics and metabolomics. Faecal microbiota transplantation (FMT) showed benefits of RS were associated with the reshaped gut microbiota composition. Monocolonization of mice with B. adolescentis, which was closely correlated with the benefits of RS in human protected mice from diet-induced obesity. Mechanistically, the RS-induced changes in the gut microbiota influenced metabolites of gut microbiome, reduced chronic low-grade inflammation by improving intestinal integrity, inhibited lipid absorption by modulating angiopoietin-like 4 (ANGPTL4), and improved the sensitivity of fibroblast growth factor 21 (FGF21) in adipose tissue. SPF, specific-pathogen-free; LPS, lipopolysaccharide; BCAAs, branched-chain amino acids; Erk1/2, extracellular signal-regulated kinase 1/2; FGFR1, fibroblast growth factor receptor 1. Created with BioRender.com.Resistant starch (RS, 40 g d-1) accompanied with isoenergetic and balanced diets led to an obvious reduction in body weight and improvement of insulin sensitivity, as well as alteration in metagenomics and metabolomics. Faecal microbiota transplantation (FMT) showed benefits of RS were associated with the reshaped gut microbiota composition. Monocolonization of mice with B. adolescentis, which was closely correlated with the benefits of RS in human protected mice from diet-induced obesity. Mechanistically, the RS-induced changes in the gut microbiota influenced metabolites of gut microbiome, reduced chronic low-grade inflammation by improving intestinal integrity, inhibited lipid absorption by modulating angiopoietin-like 4 (ANGPTL4), and improved the sensitivity of fibroblast growth factor 21 (FGF21) in adipose tissue. SPF, specific-pathogen-free; LPS, lipopolysaccharide; BCAAs, branched-chain amino acids; Erk1/2, extracellular signal-regulated kinase 1/2; FGFR1, fibroblast growth factor receptor 1. Created with BioRender.com.
Conclusions
To summarize, the study found that supplementation with resistant starch can facilitate weight loss by increasing the abundance of B. adolescentis in the gut microbiome. It can also help improve insulin sensitivity through gut microbiome-induced changes in the levels of secondary bile acids and lowering of inflammation.
Journal reference:
Li, H., Zhang, L., Li, J., Wu, Q., Qian, L., He, J., Ni, Y., KovatchevaDatchary, P., Yuan, R., Liu, S., Shen, L., Zhang, M., Sheng, B., Li, P., Kang, K., Wu, L., Fang, Q., Long, X., Wang, X., & Li, Y. (2024). Resistant starch intake facilitates weight loss in humans by reshaping the gut microbiota. Nature Metabolism. DOI: 10.1038/s4225502400988y, https://www.nature.com/articles/s42255-024-00988-y

Leave a Reply