by Levi Gadye, University of California, San Francisco
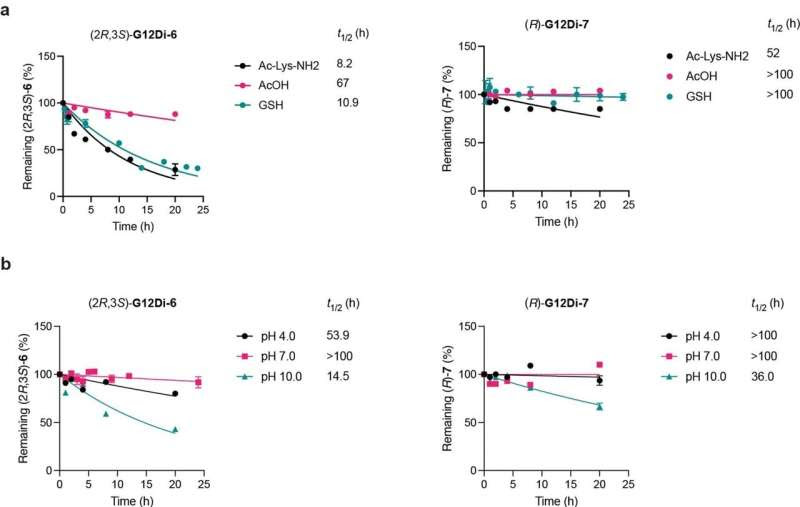
Intrinsic reactivity and stability of G12Di-6 and 7. a, Stability in the presence of excess compounds (5 mM) bearing reactive functional groups. All data points represent individual biological replicates. Data are presented as mean ± SD (n = 2). b, Stability in 1X PBS at various pH. All data points represent individual biological replicates. Data are presented as mean ± SD (n = 2).. Credit: Nature Chemical Biology (2024). DOI: 10.1038/s41589-024-01565-w
UC San Francisco researchers have designed a candidate drug that could help make pancreatic cancer, which is almost always fatal, a treatable, perhaps even curable, condition.
The new molecule permanently modifies a wily cancer-causing mutation, called K-Ras G12D, that is responsible for nearly half of all pancreatic cancer cases and appears in some forms of lung, breast and colon cancer.
Pancreatic cancer is less common than these other cancers, but the lack of treatment options makes it more deadly, and it claims more than 50,000 lives each year in the United States.
“We’ve worked for ten years to bring pancreatic cancer therapies up to speed with therapies for other cancers,” said Kevan Shokat, Ph.D., a professor in the Department of Cellular and Molecular Pharmacology who led the work. “This breakthrough is the first to target G12D and gives us a firm foothold to fight this devastating mutation.”
The findings appear in Nature Chemical Biology.
Shokat and his colleagues developed the first cancer drugs to stop a different K-Ras mutation, G12C, in 2013. Since then, two therapies have been approved for use in lung and breast cancer, but the advance didn’t move the needle for treating pancreatic cancer.
An extremely common mutation
K-Ras mutations are extremely common in pancreatic cancer, explaining 90% of cases. About half of these mutations are G12D, which differs from most other K-Ras mutations by a single amino acid substitution.
This slight difference between healthy and cancer-causing proteins, in which glycine (G) becomes aspartate (D), presented a monumental challenge for chemists.
“There are very few molecules out there that can sense the difference between the cancer-causing aspartate and the glycine,” Shokat said. “To make good therapies, we need drugs that work on the tumor cells only, without affecting healthy cells.”
Shokat’s team envisioned a molecule that fit into a pocket of the K-Ras protein, then firmly—and irreversibly—bound to the rogue aspartate. The explosion of research that followed Shokat’s 2013 discovery enabled them to develop a template for chemicals that reliably found their way into that corner of the protein.
“Once we had that structure for our molecules, we knew they were sitting in the protein at the right spot,” Shokat said. “Then we could explore the little nooks and crannies that we needed to discover the chemistry of the aspartate.”
Could a bend in a molecule lead to a cure?
The scientists went through dozens of chemicals.
“It’s like climbing a new route on a mountain; you may be strong, but the lengths of your arms limit what you can do,” Shokat said. “It was a lot of trial and error, tweaking the branches of these molecules to position them in this incredibly tight space around G12D. Some got close, then failed, and we would start over.”
Eventually, they found a winning molecule. It settled into the appropriate corner of K-Ras and bent into a new shape that reacted strongly with the aspartate.
The molecule put the brakes on tumor growth from G12D in cancer cell lines, as well as an animal model of human cancer. And it never attacked healthy proteins.
The scientists are now optimizing the molecule to be durable enough to fight cancer in the human body. With the traction gained from this study, Shokat said, new therapies for pancreatic cancer could enter clinical trials in as little as two to three years.
“We’ve learned a lot from other targeted therapies and know how to translate discoveries like these for the clinic quickly,” said Margaret Tempero, MD, director of the UCSF Pancreas Center. “An effective drug targeting K-RAS G12D could be transformative for patients with pancreatic cancer.”
More information: Qinheng Zheng et al, Strain-release alkylation of Asp12 enables mutant selective targeting of K-Ras-G12D, Nature Chemical Biology (2024). DOI: 10.1038/s41589-024-01565-w
Journal information: Nature Chemical Biology
Provided by University of California, San Francisco

Leave a Reply