by Ingrid Fadelli, Medical Xpress
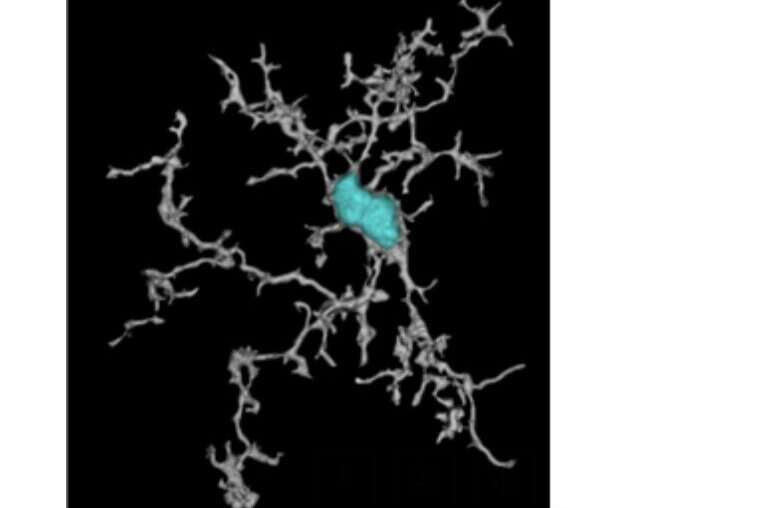
Images showing the architecture of our ATV:TREM2 molecule (a) images showing elevation of microglia proliferation in the brain of mice treated with ATV:TREM2 (b) a super resolution image of microglia caught in the act of proliferation, shown here is microglia in vivo in cytokinesis phase post ATV:TREM2 dosing; cell nuclei are labeled in teal, microglia morphological rendering in grey (c). Credit: van Lengerich et al
The protein TREM2, which in humans is encoded by the TREM2 gene, is known to play a crucial role in the growth and development of different types of immune cells, including microglia. Microglia are specialized immune cells in the central nervous system that help to protect the brain from inflammation and facilitate its recovery after injuries.
Researchers at Denali Therapeutics in South San Francisco, the German Center for Neurodegenerative Diseases (DZNE), University Hospital of Munich, Ludwig Maximilians University and Takeda have recently identified an antibody that can activate the TREM2 receptor in humans, thus potentially improving the functioning of these crucial brain immune cells. In a series of preclinical experiments, the results of which were reported in Nature Neuroscience, this engineered antibody was found to boost both the metabolism and function of microglia.
“TREM2 is the best validated microglial target for AD from a genetics and biology standpoint,” Kathryn Monroe and Pascal E. Sanchez, lead senior authors of the study, told Medical Xpress. “The totality of the human genetics and preclinical data indicate that loss-of-function of the microglial gene TREM2 increases risk of Alzheimer’s Disease, therefore activating the receptor could be an efficacious therapeutic strategy. However, the blood brain barrier limits the brain uptake and biodistribution of antibodies targeting TREM2.”
Co-first authors Bettina van Lengerich, Lihong Zhan, and Dan Xia and their collaborators explored whether a transport vehicle (i.e., engineered binding site used to transport molecules into the brain) that they developed could be used to deliver an anti-TREM2 antibody to the brain (ATV:TREM2). In addition, they wished to assess the effects of ATV:TREM2 on the function and metabolism of microglia.
“We conducted studies to demonstrate the functional impact of pharmacological activation of TREM2 on microglia and dissect out the molecular pathways by which ATV:TREM2 induces changes in microglia state,” Sanchez and Monroe explained. “We found that ATV:TREM2 can boost metabolism and the function of microglia using a multi-dimensional approach in a variety of preclinical systems.”
ATV:TREM2, the molecule tested by the researchers, is designed to bind to the protein TREM2 in cells of the human brain. It was engineered to include a monovalent human transferrin receptor binding site (i.e., the antibody transport vehicle) in the Fc region and mutations that would reduce interactions with Fc receptors, receptors in the brain that bind to antibodies attached to infected cells or invading pathogens.
The researchers tested ATV:TREM2 on human induced pluripotent stem cell (iPSC) derived microglia. They found that they promoted the proliferation of these crucial immune cells and improved their metabolism. They also tested their antibody in mice and found that it significantly boosted microglial activity and glucose metabolism in the animals’ brain.
“Our study offers insights into TREM2 antibody mechanisms of action by inducing TREM2-TfR receptor clustering and the ATV’s ability to enhance TREM2 Fab-mediated signaling activity,” Monroe said. “We provide a deeper understanding of the pharmacological impact of ATV:TREM2 on microglia state and function, such as boosting microglia metabolism and mitochondrial respiration.”
Overall, the researchers’ experiments showed that ATV:TREM2 could improve brain exposure, biodistribution and agonistic activity in the brain, significantly outperforming standard IgG TREM2 antibodies. This suggests that it could be a promising therapeutic for patients affected by Alzheimer’s disease and other neurodegenerative diseases that have been linked with a loss-of-function of the microglial gene TREM2.
“We are now conducting a single ascending dose Phase 1 study of DNL919, our investigational ATV:TREM2, in healthy volunteers,” Sanchez and Monroe added. “Additionally, we are interested in studying whether ATV:TREM2 has additive or synergistic effects with anti-amyloid antibody treatments.”

Leave a Reply