By Suchandrima Bhowmik Dec 28 2021 Reviewed by Aimee Molineux
Several studies have indicated an association between COVID-19 vaccination and myocarditis. In addition, evidence from several countries suggested exposure to the BNT162b2 messenger RNA (mRNA) vaccine was associated with acute myocarditis.
Furthermore, an increase in hospitalization or death due to myocarditis has been reported against both mRNA and adenoviral vaccines.
However, across the entire vaccinated population in England, the risk of myocarditis following vaccination was small compared to the risk following a positive severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) test.
Myocarditis was reported to be more common in younger individuals and males. However, since the risk of myocarditis is higher after the second dose of vaccine as compared to the first dose, there is an immediate requirement to evaluate the risk associated with a third dose as booster programs are being implemented internationally to combat the Omicron variant of SARS-CoV-2.
A new study posted to the medRxiv* pre-print server included individuals over 13 years of age receiving the third vaccine dose to further evaluate the association between myocarditis and COVID-19 vaccination or infection. The study took place from December 1, 2020, to November 15, 2021.

Study: Risk of myocarditis following sequential COVID-19 vaccinations by age and sex. Image Credit: Kateryna Kon / Shutterstock
About the study
The study utilized the NHS Immunisation Management Service (NIMS) database to gather data on all individuals who received the COVID-19 vaccine in England.
To determine the association between the first, second, or third dose of ChAdOx1 (AstraZeneca), BNT162b2 (Pfizer), or mRNA-1273 (Moderna) vaccine or SARS-CoV-2 infection with myocarditis, individual patient data were matched to national data for mortality, hospital admission, and SARS-CoV-2 testing.
Researchers used a self-controlled case series (SCCS) design originally developed to evaluate vaccine safety. An incidence rate ratio (IRR) was calculated for deaths or hospitalizations in a 1-28 day risk period after vaccination or a positive test. Additionally, the IRR was calculated after stratification by sex and age in individuals under or over 40. Individuals hospitalized with myocarditis two years before the study period and those who received the Ad26.COV2.S (Janssen) vaccine were excluded.
Study findings
According to the results, 13.5 percent of the individuals tested positive for SARS-CoV-2 after receiving the first dose of the vaccine, 30.9 percent after receiving the second dose, and 0.9 percent after receiving the third dose. The percentage of individuals hospitalized or who died due to myocarditis during the study period was 0.006 out of which 0.001 percent occurred within 1-28 days following any dose of vaccine.
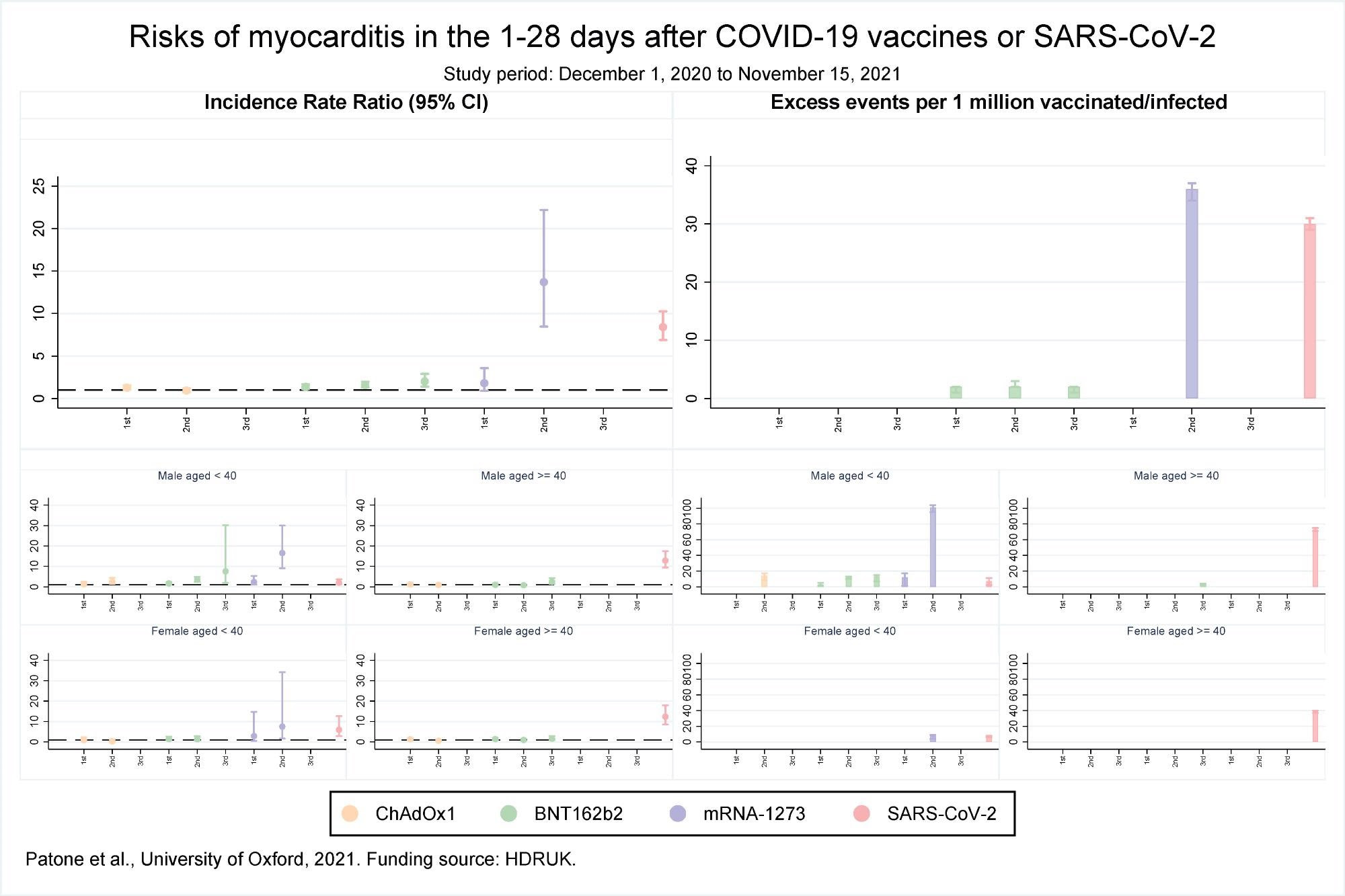
Risks of myocarditis in the 1-28 days after COVID-19 vaccines or SARS-CoV-2
The results reported that over the 1 to 28 days post-vaccination, an association was observed with the first dose of BNT162b2 and ChAdOx1 but not the mRNA-1273 vaccine. However, after the second dose, the risk of association was higher with mRNA-1273 as compared to the BNT162b2 vaccine. No association with the ChAdOx1 vaccine was found after the second dose. Association with only the BNT162b2 vaccine was observed after the third dose. The risk for myocarditis was also higher in the 1 to 28 days following a positive SARS-CoV-2 test.
An increased risk of myocarditis was observed in men less than 40 years of age on days 1-28 following the first dose of mRNA-1273 and BNT162b2 vaccines, second dose of ChAdOx1, mRNA-1273, and BNT162b2 vaccines, third dose of BNT162b2 vaccine, and a SARS-CoV-2 positive test. In females, an increased risk of myocarditis was observed in 1-28 days following the second dose of the mRNA-1273 vaccine only. In the case of older women, no association between myocarditis and vaccination was observed while in older men the risk of myocarditis increased after the third dose of BNT162b2 vaccine and a positive SARS-CoV-2 test.
An estimate of the number of excess myocarditis events per million persons in the 1-28 days following exposure showed there would be an additional 1 and 2 myocarditis events per million exposed following the first dose of the ChAdOx1 and BNT162b2 vaccines, respectively. Approximately 2 and 36 myocarditis events could be expected after the second dose of BNT162b2 and mRNA-1273 vaccines, respectively, while an additional 2 myocarditis events per million persons could be expected after the third dose of BNT162b2. Following a positive test additional 30 myocarditis events per million could be expected.
An additional 3 and 12 myocarditis events per million in the 1-28 days were estimated in males less than 40 years of age following the first dose of BNT162b2 and mRNA-1273 vaccines respectively. An additional 14,12 and 101 myocarditis events could be estimated after the second dose of ChAdOx1, BNT162b2, and mRNA-1273 vaccines respectively, and an additional 13 events following the third dose of BNT162b2 vaccine. An additional 7 myocarditis events could be expected after a positive SARS-CoV-2 test. For older males, 3 and 73 events could be estimated following a third dose of BNT162b2 and a positive SARS-CoV-2 test respectively.
For females, less than 40 years, an additional 8 and 7 myocarditis events could be expected following the second dose of mRNA-1273 vaccine and a positive SARS-CoV-2 test, respectively. For older females, an additional 39 events per million could be expected following a positive SARS-CoV-2 test.
Therefore, the current study shows that the risk of death or hospitalization from myocarditis is higher following the first and second doses of vaccination. In contrast, the risk is significantly lower following the third vaccination dose. Furthermore, compared to adenovirus vaccines, mRNA vaccines had a higher risk. Additionally, the study indicated that myocarditis risk was higher in males under 40 years of age than females of the same age group.
Limitations
There were some limitations to the study. First, the number of individuals receiving the mRNA-1273 or ChAdOx1 vaccine was too few to evaluate the risk of myocarditis. Due to the study relying on hospital admission codes and death certificates to determine myocarditis, there is the potential for overestimation or underestimation of risk. Third, myocarditis in children ages 13 to 17 years was less which could alter the risk evaluation.

Leave a Reply