by Columbia University
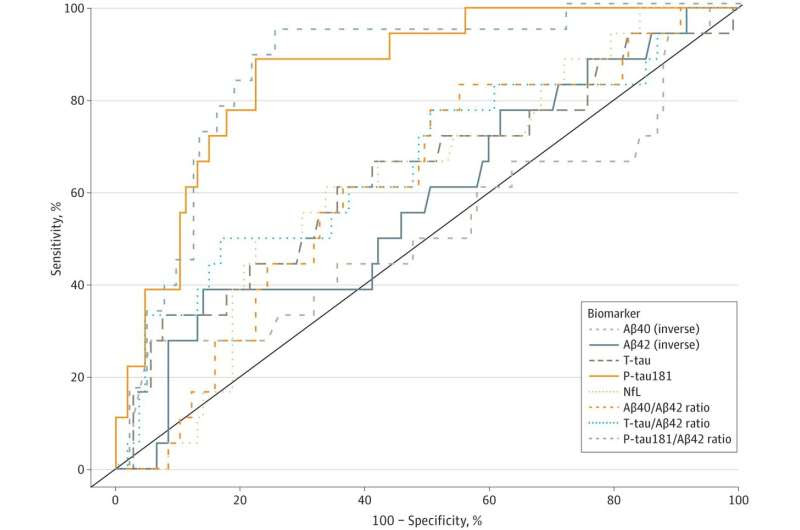
Receiver Operator Curve Analyses of Plasma Biomarker Performance in Classifying Cerebrospinal Fluid (CSF)–Supported Diagnosis of Alzheimer Disease Performance of plasma biomarkers amyloid-β 1-40 (Aβ40) (inverse), amyloid-β 1-42 (Aβ42) (inverse), total tau (T-tau), neurofilament light chain (NfL), phosphorylated tau181 (P-tau181), and ratios of Aβ40/Aβ42, T-tau/Aβ42, and P-tau181/Aβ42 in classifying CSF biomarker (P-tau181/Aβ42)–supported diagnosis of biological Alzheimer disease. Credit: JAMA Network Open (2023). DOI: 10.1001/jamanetworkopen.2023.8214
Columbia University neurologists are investigating a set of blood tests that, used in combination with memory tests, may help physicians correctly diagnose Alzheimer’s disease in low-resource environments, where 58% of people worldwide living with dementia reside.
Low-resource communities often lack specialists who can diagnose Alzheimer’s disease or do not have access to brain imaging machines that can confirm a diagnosis of Alzheimer’s disease. Biomarkers in spinal fluid can confirm a diagnosis, but patients may not wish to have a lumbar puncture to remove cerebrospinal fluid.
In recent years, blood-based biomarkers have been found that perform almost as well as biomarkers in spinal fluid in identifying people with Alzheimer’s disease. Few studies have been conducted in low-resource communities to determine if the blood tests could help physicians accurately identify individuals with Alzheimer’s disease.
In a new study, Columbia researchers in collaboration with local physicians recruited 746 older adults (average age of 71 years) from the Dominican Republic and the Dominican community of northern Manhattan.
Each participant underwent detailed neurological and cognitive testing. A panel of Columbia neurologists, expert in Alzheimer’s diagnosis, analyzed the results to judge whether participants had Alzheimer’s disease, other forms of dementia, or normal cognitive aging. Blood was drawn from each participant to measure blood-based biomarkers for Alzheimer’s disease.
Among the study participants, just over 20% were given a clinical diagnosis of Alzheimer’s disease. The blood tests showed that these individuals also had higher levels of Alzheimer’s biomarkers (including phosphorylated tau, neurofilament light chain, and glial fibrillary acidic protein).
Remarkably, these blood-based tests also may identify the earliest biological signs of Alzheimer’s disease before the onset of symptoms. In this study, about 20% of participants who were not diagnosed with the disease based on their performance on memory and cognitive tests had elevations of phosphorylated tau, neurofilament light chain, and glial fibrillary acidic protein in their blood.
This finding suggests that the blood tests may be a sensitive indicator of preclinical Alzheimer’s disease. As more preventive strategies become available, early recognition in the preclinical stage would be beneficial.
“Alzheimer’s disease is one form of dementia and as more treatments emerge, developing an accurate blood test will be essential in finding people who will benefit from treatment, particularly those who lack access to more advanced diagnostic tools,” says Richard Mayeux, MD, chair of the Department of Neurology at Columbia University Vagelos College of Physicians and Surgeons and one of the study’s senior authors.
More research is needed to determine how the blood-based biomarker tests can be used in the clinic; the tests are not currently available outside of research studies.
The findings are published in the journal JAMA Network Open.

Leave a Reply