Bone marrow-derived mesenchymal stem cells (MSCs) can be induced to make cartilage by incubating the cells with particular growth factors. Unfortunately, batches of MSCs show respectable variability from patient-to-patient. Therefore the growth factor-dependent method suffers from poor efficacy, limited reproducibility from batch-to-batch, and the cell types that are induced are not always terribly stable. Finding a better way to make cartilage would certainly be a welcome addition to regenerative treatments,
Cartilage that coats the ends of bones is known as articulate cartilage, and articular cartilage lacks blood vessels. Therefore, is it possible that inhibiting blood vessel formation could conveniently push MSCs to differentiate into cartilage-making chondrocytes?
A new paper by Ivan Martin and Andrea Basil from the University Hospital Basel and their colleagues have used this very strategy to induce cartilage formation in MSCs from bone marrow.
Martin and others isolated MSCs from bone marrow aspirates from human donors. These cultured human MSCs were then genetically engineered with modified viruses to express a receptor for soluble vascular endothelial growth factor (VEGF) that binds this growth factor, but fails to induce any intracellular signals. Such a receptor that binds the growth factor but does not induce any biological effects is called a “decoy receptor,” and decoy receptors efficiently sequester or vacuum up all the endogenous VEGF. VEGF is the major blood vessel-inducing growth factor and it is heavily expressed during development, by cancer cells, and during healing.
After expressing the decoy VEGF receptor in these human MSCs, these genetically engineered cells were grown on collagen sponges and then implanted in immunodeficient mice. If the implanted MSCs were not genetically engineered to express decoy VEGF receptors, they induced for formation of vascularized fibrous tissue. However, the implantation of genetically engineered MSCs that expressed the decoy VEGF receptor efficiently and reproducibly differentiated into chondrocytes and formed hyaline cartilage. This is significant because headline cartilage is the very type of cartilage found at articular surfaces where the ends of bones come together to form joints.
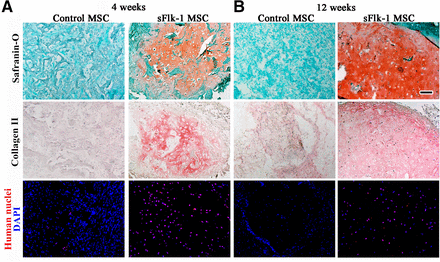
This articular cartilage was quite stable and showed no signs of undergoing the chondrocytes enlargement found in terminally differentiated cartilage that is ready to form bone. This stability was maintained for up to 12 weeks.

Why did inhibition of VEGF signaling induce cartilage? Inhibition of angiogenesis induced low oxygen tensions, which activated a growth factor called transforming growth factor-β. Activation of the TGF-beta pathway robustly enhanced the formation of articular cartilage.
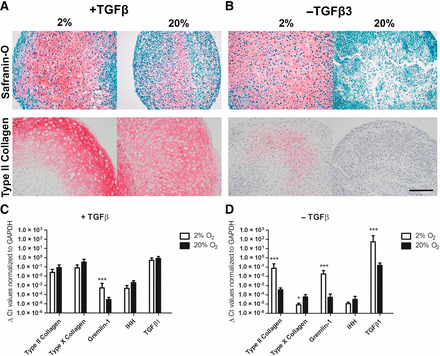
Cartilage formation from MSCs was induced by blocking VEGF-mediated angiogenesis. These results represent a remarkable advance in cartilage formation that can be used for regenerative treatments. This cartilage formation was spontaneous and efficient and if it can be carried out with VEGF-inhibiting drugs rather than genetic engineering techniques, then we might have a transferable technique for making cartilage in the laboratory to treat osteoarthritis and other joint-based maladies. Clinical trials will be required, but this is certainly an auspicious start.
