After decades of frustration and failed attempts, scientists might finally be on the cusp of developing therapies to restore immune ‘tolerance’ in conditions such as diabetes, lupus and multiple sclerosis.
Cassandra Willyard
Antigen presenting cells (illustrated, right) can sometimes train T cells (left) not to attack. Credit: Juan Gaertner/Science Photo Library
Back in 2001, immunologist Pere Santamaria was exploring a new way to study diabetes. Working in mice, he and his collaborators developed a method that uses iron oxide nanoparticles to track the key immune cells involved in the disorder.
But then Santamaria, who is at the University of Calgary in Canada, came up with a bold idea. Maybe he could use these particles as a therapy to target and quiet, or even kill, the cells responsible for driving the disease — those that destroy insulin-producing islet cells in the pancreas. It seemed like a far-fetched idea, but he decided to try it. “I kept doing experiment after experiment,” he says. Now, more than two decades later, Santamaria’s therapy is on the cusp of being tested in people.
It’s not alone. Researchers have been trying for more than 50 years to tame the cells that are responsible for autoimmune disorders such as type 1 diabetes, lupus and multiple sclerosis. Most of the approved therapies for these conditions work by suppressing the entire immune response. This often alleviates symptoms but leaves people at elevated risk of infections and cancers.
But for decades, immunologists have hoped to restore what’s known as tolerance — the immune system’s ability to ignore antigens that belong in the body while appropriately attacking those that don’t. In some cases, that means administering the very antigens that the rogue cells are trained to attack, a strategy that can deprogram the cells and dampen the autoimmune response. Other researchers are trying to selectively wipe out the problematic cells, or to introduce suppressive immune cells that have been engineered to target them. One approach that relies on engineered immune cells was used to treat 15 people with lupus or other immune disorders with surprising success1. One participant has been symptom-free for more than two and a half years.
If more trials in people show positive results, it could change lives, says Maximilian Konig, a rheumatologist at Johns Hopkins University in Baltimore, Maryland, who specializes in autoimmune disorders. “The therapies that we have for these diseases are terrible,” he says. They don’t always work, and even when they do, the improvement is often modest. Talking about therapies that could provide a cure is “almost blasphemy”, Konig says. Now, with some of the results coming in, curative treatments seem possible. “The only question is, what is the best approach?” he asks.
Self awareness
The immune system is known for its role in attacking pathogens. But it has another, equally important job: knowing when to stand down. If immune cells see the body’s own tissues as a threat, they can cause harm.
The arm of the immune system engaged in targeted attacks consists of cells called lymphocytes that differentiate to form T cells, which typically recognize and attack foreign or diseased cells, and B cells, which produce antibodies and support the immune system in other ways. Early in their development, these cells undergo a process to weed out those that target the body’s own tissues. But this process, known as central tolerance, is “leaky”, says Jeffrey Hubbell, a chemical engineer at the University of Chicago in Illinois. Some cells slip through.
‘It’s all gone’: CAR-T therapy forces autoimmune diseases into remission
The body has a backup system, a mechanism known as peripheral tolerance. This eliminates or deactivates wayward cells, or turns them into what are known as regulatory T cells, which suppress the immune response by preventing other cells from attacking.
In autoimmune diseases, both central and peripheral tolerance break down for reasons that are not entirely understood, and the immune system starts attacking antigens found on the body’s own cells and tissue. In multiple sclerosis, the body attacks the myelin sheath that insulates nerves. In coeliac disease, gluten triggers the immune system to attack the intestinal lining.
If scientists could calm or eliminate the malfunctioning cells — restoring tolerance to specific antigens — they could treat the disease without hampering the immune system’s ability to respond to real threats. Early efforts focused on administering large quantities of the problematic antigen in an attempt to exhaust or deactivate the immune cells that recognize it. Many groups have tried this form of desensitization, with varying levels of success. The antigen must be introduced in just the right way, to boost tolerance rather than activate immunity, says Jeffrey Bluestone, an immunologist and chief executive and co-founder of Sonoma Biotherapeutics in South San Francisco, California. “That’s the whole field of antigen-specific therapy.”
Flipping the switch
Picking the right antigen is tricky. Some autoimmune diseases are caused by a reaction to just one. But for many conditions — multiple sclerosis, diabetes and rheumatoid arthritis, to name a few — the body develops an immune response against several antigens through a phenomenon called epitope spreading. Researchers could try to work out all the antigens involved, Bluestone says. But that’s a tall order, especially because the list might vary from person to person. Another possibility is to search for a master switch that, when flipped, turns off the autoimmune response while leaving the immune system intact.
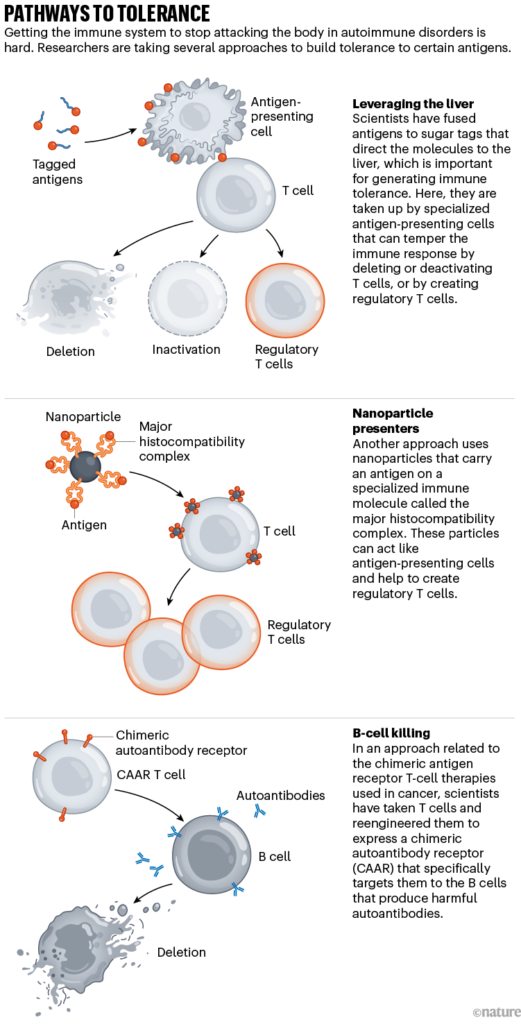
Santamaria thinks that he has found such a mechanism. His team’s nanoparticles — which he calls navacims — are decorated with proteins called major histocompatibility complex molecules that jut off the surface of the particle like spikes. These spikes are meant to mimic those found on immune regulatory cells known as antigen-presenting cells, which mop up antigens all over the body and then take them to T cells, telling the cells whether to attack or ignore them (see ‘Pathways to tolerance’). Santamaria likens them to T-cell bait.
Pathways to tolerance. Diagram illustrating three different treatment approaches.
Sources: Liver: Ref. 4; Nanoparticles: Y. Yang & P. Santamaria Adv. Drug Deliv. Rev. 176, 113898 (2021); CAAR: Ref. 6
After Santamaria discovered how to use the navacims to track T cells, he began working out how to use them as a therapy. He suspected that the nanoparticles might cause the cells to die or go dormant.
At the time, Santamaria was working with a mouse model of type 1 diabetes, a disease in which epitope spreading is known to occur. So, he developed a cocktail of particles carrying eight different antigen fragments, or epitopes. For a control, he used a nanoparticle carrying a single epitope, surmising that one wouldn’t have any effect because T cells recognize thousands of epitopes in diabetes. Surprisingly, however, both the control and the experimental nanoparticles reversed diabetes symptoms. “It totally didn’t make any sense,” Santamaria says.
It took him years to work out what was going on, but now he thinks he understands2,3. The nanoparticles prompt T cells to multiply and morph into regulatory T cells, which travel to the site of inflammation. There, they bind and deactivate antigen-presenting cells that carry not only the antigen that these cells recognize, but thousands of other antigens that are important in diabetes. So these cells can no longer activate the immune cells that fuel the disease. In a sense, the nanoparticle-fused antigen works as a master switch. “It sounds too good to be true sometimes,” Santamaria says.
And it might be. The strategy hasn’t been tested in the clinic yet. Santamaria has founded a company called Parvus Therapeutics in South San Francisco; it should begin its first trial in people this year, starting with an autoimmune disease that affects the liver.
A key in the liver
The liver is where all the blood carrying foreign antigens from the gut gets filtered. It is also the destination of all the cellular debris left behind when cells and tissues die. It, therefore, has an important role in establishing immune tolerance. Hubbell and his colleagues discovered that cellular debris carries a special sugar tag that directs it to the liver. By adding this sugar tag to other proteins, they realized, they could direct just about any molecule they wanted to the liver, including antigens such as the myelin proteins that activate the immune system in multiple sclerosis. In work published in 2023, they showed that this strategy works to reverse symptoms of a multiple-sclerosis-like disease in mice4.
What was so exciting about this paper, Hubbell says, is that the animals had advanced disease, meaning that their immune systems were probably reacting against a variety of antigens. Yet, treatment with just one antigen worked to reverse paralysis.

Your brain could be controlling how sick you get — and how you recover
A company called Anokion in Cambridge, Massachusetts, which Hubbell co-founded, is working on a similar strategy. It has tested it in a phase I trial in people with multiple sclerosis to assess its safety, and is currently enrolling participants in a phase II trial, in which it will start to assess the efficacy. The firm’s chief executive, Deborah Geraghty, won’t say exactly how the therapy works after the antigen travels to the liver. “We haven’t disclosed a lot of that publicly,” she says, but she adds, “We believe we’re driving a strong T-regulatory component.”
Bluestone has decided on a different approach. He and his colleagues at Sonoma Biotherapeutics take T cells from patients’ blood, extract the regulatory cells, and engineer them to express an antigen that will direct the regulatory T cells to the site of disease when they are re-injected. There, they should be able to calm all the angry T cells in the vicinity, not just those that recognize the specific antigen they carry.
Sonoma has tested regulatory T cells in clinical trials, but not those cells engineered to express a specific antigen, yet. It plans to give the first dose to a person with rheumatoid arthritis in early 2024.
Bluestone thinks that the approach is less risky than dosing people with antigens that might further exacerbate autoimmunity. And regulatory T cells produce tissue-repair factors, which might help to reverse some of the damage wrought by disease. But cellular therapies also come with challenges, including a high cost and potential side effects. “These are living drugs,” Bluestone says. How do you determine a dose for cells that, once infused, begin replicating in the body? “There’s a lot we still need to learn.”
B cells in the spotlight
While researchers such as Bluestone and Hubbell are working to prod the immune system towards tolerance, others are developing therapies to directly kill the immune cells that contribute to the disease.
One team in Germany is trying an approach commonly used to treat blood cancers that equips T cells with what’s known as a chimeric antigen receptor (CAR). Clinicians take T cells from the patient’s body and engineer them to express this specialized synthetic receptor on their surface. They can create a CAR that binds to CD-19, a protein found on all B cells. Clinicians then infuse the CAR T cells into the body, where they start attacking B cells.

How a pioneering diabetes drug offers hope for preventing autoimmune disorders
So far, the researchers have presented results for a total of 15 people: 8 with lupus; 4 with systemic sclerosis, which affects connective tissue; and 3 with idiopathic inflammatory myositis, which causes muscle inflammation and weakness. People’s symptoms improved dramatically. But what’s most surprising is that they have all stayed in remission, even after they began producing new B cells. It’s as if wiping out the B cells performed a reset on the immune system5.
“The results are mind blowing, boggling, outstanding,” says Lawrence Steinman, a neuroimmunologist at Stanford University in California, who many call the father of antigen-specific therapy. The first participant in the study, who had lupus, passed the 1,000-day mark in December with no sign of disease. Konig agrees that the results are “incredible”:“Most people would not have predicted the degree of durability.” But, he adds, “I don’t think we can expect that the disease will stay gone forever in every patient.” The T cells that help to drive lupus are still there.
CAR-T therapies target and kill all B cells, which might make it a stretch to consider the approach a way of restoring tolerance. But for most autoimmune diseases, only a small fraction of B cells are the trouble makers. Aimee Payne, a dermatologist at Columbia University Vagelos College of Physicians and Surgeons in New York City, wanted to find a way to target just those cells. She studies a rare skin disease called mucosal pemphigus vulgaris. In people with this disease, the immune system makes antibodies against a protein in the skin called desmoglein 3. “It’s responsible essentially for holding your epithelial cells together,” she says. “The cells fall apart and you get blisters all throughout the mucosa [and] on the skin.”
The standard therapy for pemphigus, a monoclonal antibody, works by destroying B cells. With the B cells gone, the antibodies against desmoglein 3 disappear, and people go into remission. “Our current therapies are really a blunt weapon,” Payne says.
Payne realized that she might be able to develop a more focused weapon by tweaking the CAR-T approach. Rather than engineering a T-cell receptor that would bind to all B cells, she could engineer the T cells to express the desmoglein 3 protein, which would bind to the desmoglein 3 antibodies sticking off the surface of only the rogue B cells6. This therapy is known as CAAR (chimeric autoantibody receptor) T cell. “It’s like a laser,” she says, “targeting the autoreactive B cells that are causing disease, but leaving the healthy B cells alone.”
The race to supercharge cancer-fighting T cells

Payne founded a company called Cabaletta Bio in Philadelphia, Pennsylvania, and is currently testing these cells in people with pemphigus and another autoimmune disease called myasthenia gravis that causes muscle weakness.
B cells are emerging as a promising target in many autoimmune diseases, says Payne. In some of the most common disorders, B cells have complex roles. They don’t just produce antibodies but also churn out signals that trigger inflammation and present antigens to T cells, helping to fan the flames that keep autoimmune diseases burning. In immunology, “everybody focused on T cells because they were the smart ones and B cells were just like the poor cousin”, Payne says, but “maybe B cells really are like the key”.
Steinman is careful not to be too optimistic. He has invested decades in approaches directed at immune tolerance. “Every time these are taken to the clinic, including some of my own efforts, they fall short of getting a drug approval,” he says. At this point, he adds, “I would want to hedge my bets.”
Santamaria suggests that many past failures stem from a poor understanding of the underlying mechanisms. “I think they fail because they’re going to into clinical testing before we actually know what the hell we’re doing,” he says. Now, 18 years into his research on navacims, he thinks that his team has a good shot at success.
Konig favours approaches that involve B-cell depletion. That’s the strategy that his team is working on, so he accepts that he might be biased. “The beauty is that eventually we’ll learn from the data what the right approach is.”
Nature 625, 646-648 (2024)
doi: https://doi.org/10.1038/d41586-024-00169-7
References
Mueller, F. et al. Blood 142, 220 (2023).
Article
Google Scholar
Tsai, S. et al. Immunity 32, 568–580 (2010).
Article
PubMed
Google Scholar
Solé, P. et al. Cell. Mol. Immunol. 20, 489–511 (2023).
Article
PubMed
Google Scholar
Tremain, A. C. et al. Nature Biomed. Eng. 7, 1142–1155 (2023).
Article
PubMed
Google Scholar
Mackensen, A. Nature Med. 28, 2124–2132 (2022).
Article
PubMed
Google Scholar
Ellebrecht, C. T. et al. Science 353, 179–184 (2016).
Article
PubMed
Google Scholar

Leave a Reply