August 16, 2024 The U.S. Food and Drug Administration has approved Galderma’s Nemluvio (nemolizumab) for adult patients living with prurigo nodularis. Nemluvio, administered as a prefilled pen for subcutaneous injection, inhibits interleukin-31 cytokine signaling, which is known to drive itch and is involved in inflammation, altered epidermal differentiation, and fibrosis in prurigo nodularis. The approval...
Category: <span>Pharmaceutical Updates</span>
Sexual Arousal Cream Promising in Some Subsets of Women
MDedge News Marcia Frellick August 14, 2024 Topical sildenafil (citrate) cream 3.6% used by healthy premenopausal women with a primary symptom of female sexual arousal disorder did not show statistically significant improvement over placebo in the coprimary or secondary endpoints over a 3-month period in new preliminary study data published in Obstetrics & Gynecology. Topical...
Study finds that dopaminergic medication improves sleep quality in Parkinson’s disease patients
August 13, 2024 by FAPESP Credit: Pixabay/CC0 Public DomainA study involving 22 Parkinson’s disease (PD) patients has shown that use of the dopaminergic drug levodopa improves sleep quality. When the patients took the drug, the number of times they woke up during the night fell 25% and the amount of time they remained awake fell...
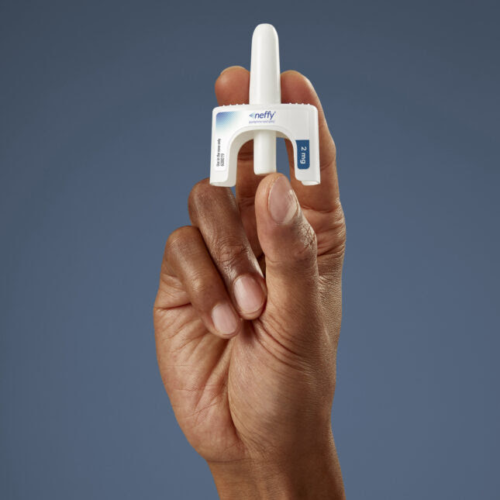
FDA approves EpiPen alternative, a nasal spray for anaphylaxis
Pharma By Isabella Cueto Aug. 9, 2024 Neffy, the new nasal spray for severe allergic reactions, is approved for use in people 66 pounds and above.Courtesy ARS Pharma On Friday, the Food and Drug Administration approved the first needle-free treatment for adults and kids with severe allergic reactions. The approval introduces a competitor from ARS...
Statin prescription can reduce the risk of cardiovascular diseases against air pollutant exposure in older adults
August 9, 2024 by Korea University College of Medicine Graphical Abstract. Credit: European Journal of Preventive Cardiology (2024). DOI: 10.1093/eurjpc/zwae061It has been discovered that older adults over 60 years old who are prescribed statins against air pollutant exposure can reduce the risk of cardiovascular disease, especially stroke. By utilizing the National Health Insurance Service big...
Biocides are a useful tool to combat antibiotic resistance but appropriate use is vital, scientists suggest
News Release 8-Aug-2024 Peer-Reviewed PublicationApplied Microbiology International A recent review in the journal Sustainable Microbiology discusses how the use of biocides can promote well-being – but must only be used when there are clear benefits. Biocide use should be restricted to applications where there are tangible benefits but also not unnecessarily restricted where genuine benefits...
Common antibiotics carry small but serious risks of life-threatening drug reactions, but some are safer than others
News Release 8-Aug-2024 Peer-Reviewed PublicationInstitute for Clinical Evaluative Sciences image: Common antibiotics carry serious riskof life-threatening drug reactions,but safer options exist Credit: ICES Toronto, ON, May 15, 2024 – Two classes of commonly prescribed oral antibiotics are associated with the greatest risk for severe drug rashes that can lead to emergency department visits, hospitalizations and...
FDA approves Darzalex Faspro for treating multiple myeloma
August 8, 2024 by Lori Solomon The U.S. Food and Drug Administration has approved Johnson & Johnson’s Darzalex Faspro (daratumumab and hyaluronidase-fihj) in combination with bortezomib, lenalidomide, and dexamethasone (D-VRd) for induction and consolidation in patients with newly diagnosed multiple myeloma (MM) who are eligible for an autologous stem cell transplant (ASCT). The approval was...
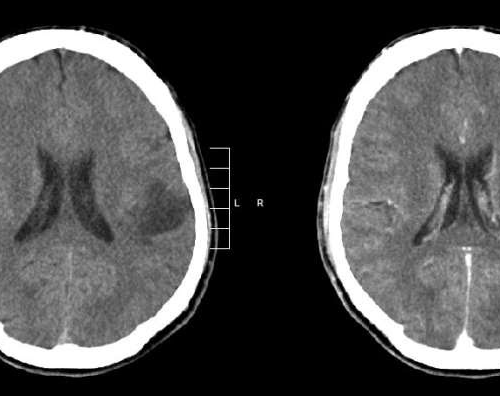
FDA approves new therapy for glioma patients for first time in decades
August 7, 2024 by Dana-Farber Cancer Institute Glioma of the left parietal lobe. CT scan with contrast enhancement. Credit: Mikhail Kalinin/CC BY-SA 3.0Vorasidenib has been approved by the U.S. Food and Drug Administration (FDA) for patients with Grade 2 gliomas with IDH1 or IDH2 mutations. Based on evidence from the INDIGO clinical trial, a global...
Semaglutide products being sold online without prescriptions
August 3, 2024 by Elana Gotkine Semaglutide products are being sold online, with products likely unregistered or unlicensed, according to a research letter published online Aug. 2 in JAMA Network Open. Amir Reza Ashraf, Pharm.D., from the University of Pécs in Hungary, and colleagues conducted a risk assessment of semaglutide online sourcing. Websites advertising semaglutide...