News Release 9-Sep-2024
The review explores how biomarkers could revolutionize the diagnosis, prognosis, and treatment of pulmonary fibrosis for enhanced patient outcomes
Peer-Reviewed Publication
Chinese Medical Journals Publishing House Co., Ltd.
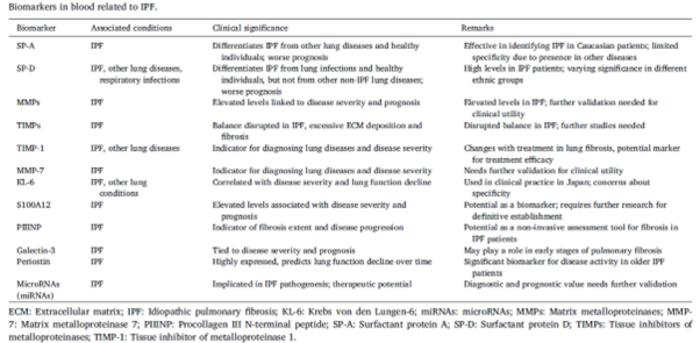
image:
Biomarkers revolutionize the diagnosis and management of Idiopathic Pulmonary Fibrosis, leading to improved patient outcomes
Credit: Yong Zhou of the Tulane University Image Source Link: https://www.sciencedirect.com/science/article/pii/S2772558824000240?via%3Dihub#tbl0001
Idiopathic Pulmonary Fibrosis (IPF) is a chronic and progressive lung disease characterized by a gradual decline in lung function and a poor prognosis. Traditionally, diagnosing IPF relied on clinical evaluations and radiographic imaging, which often resulted in delays in diagnosis and treatment due to the disease’s subtle onset. Early and accurate identification of IPF is crucial for improving patient outcomes. Recent research is focused on biomarkers, to enhance the precision of IPF diagnosis, prognosis, and management.
A research team from Tulane University, led by Professor Yong Zhou, conducted a comprehensive review exploring the background of IPF, the latest research on biomarkers, and significant findings from recent studies. Published online in the journal Chinese Medical Journal Pulmonary and Critical Care Medicine on June 26, 2024, this review examines the biomarkers’ potential to enhance early detection, more accurately predict disease progression, and personalize treatment.
Diagnosing IPF is challenging due to its heterogeneous presentation. High-resolution computed tomography (HRCT) and clinical assessments are commonly used, but they often lead to late diagnoses. Biomarkers—biological indicators found in blood, tissues, or other fluids—offer a promising solution for earlier and more precise management of IPF. This review evaluates how biomarkers can complement traditional diagnostic methods to enhance IPF diagnosis and treatment.
The study employed a comprehensive approach to biomarker research, incorporating various technologies and methodologies. It analyzed a range of biomarkers, including exhaled breath markers like volatile organic compounds (VOCs) and nitric oxide (NO), which provide non-invasive insights into lung inflammation. Bronchoalveolar lavage (BAL) and sputum biomarkers, such as cellular differentials and cytokines, assess inflammatory and fibrotic processes. Imaging biomarkers from HRCT and positron emission tomography (PET) scans offer detailed views of lung structure and fibrosis. Gene-expression profiling through RNA sequencing and microarrays uncovers molecular signatures and disease pathways, while proteomic and metabolomic biomarkers reveal disruptions in biological processes. Emerging biomarkers, including epigenetic modifications, exosomes, and microbiome changes, introduce new diagnostic and monitoring opportunities.
Prof. Zhou elucidates the findings, “Blood-based biomarkers such as Surfactant protein D (SP-D), Krebs von den Lungen-6 (KL-6), and Matrix metalloproteinases (MMPs) are particularly noteworthy. Elevated levels of SP-D and KL-6 have been linked with a worse prognosis, while increased levels of MMPs indicate heightened fibrotic activity and accelerated disease progression. Genetic variations, specifically mucin 5B (MUC5B) and telomerase reverse transcriptase (TERT), have been identified as risk factors contributing to the on-set and progression of IPF.”
Advancements in quantitative HRCT and PET/CT provided deeper insights into disease severity and fibrotic activity. Multi-omics approaches enriched the understanding of IPF’s molecular landscape, while novel biomarkers and molecular pathways identified could lead to targeted therapies and personalized treatment strategies.
The review offers various key insights and advances our understanding of IPF management. Biomarkers enhance diagnosis by enabling early and precise identification of IPF, facilitating timely interventions that could slow disease progression and improve patient outcomes. The findings also support personalized treatment by linking biomarkers to disease severity and progression, allowing for customized therapies and optimized treatment efficacy. Additionally, the study enhances our understanding of the molecular mechanisms underlying IPF, paving the way for targeted therapies and innovative treatment approaches.
“Despite the considerable advancements in biomarker research, certain challenges remain,” explains Prof. Zhou. “The standardization and reproducibility of biomarker assays are vital for ensuring consistent and dependable results across varied research endeavors and clinical settings. Moreover, it is essential to validate the clinical utility and relevance of these biomarkers through studies involving large and diverse patient cohorts.” He emphasizes, “To overcome the challenges of standardization and reproducibility in biomarker research for clinical applications, it is essential to establish rigorous standardization protocols for biomarker assays, including uniform procedures for sample collection, processing, and analysis. Integrating multiple biomarkers with clinical data could revolutionize risk stratification and treatment decision-making, marking a significant stride toward personalized medicine for IPF patients.”
In conclusion, this review underscores the game-changing potential of biomarkers in revolutionizing IPF management. By enhancing diagnostic precision and prognosis, biomarkers are steering the future toward personalized medicine, promising a new era of improved patient care and outcomes in the fight against this challenging lung disease.
Reference
Titles of original papers: Biomarkers in idiopathic pulmonary fibrosis: Current insight and future direction
Journal: Chinese Medical Journal Pulmonary and Critical Care Medicine
DOI: https://doi.org/10.1016/j.pccm.2024.04.003
DOI
10.1016/j.pccm.2024.04.003
Method of Research
Literature review
Subject of Research
Not applicable
Article Title
Biomarkers in idiopathic pulmonary fibrosis: Current insight and future direction
Article Publication Date
26-Jun-2024
COI Statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.

Leave a Reply