by Andrew J. Yawn, Tulane University
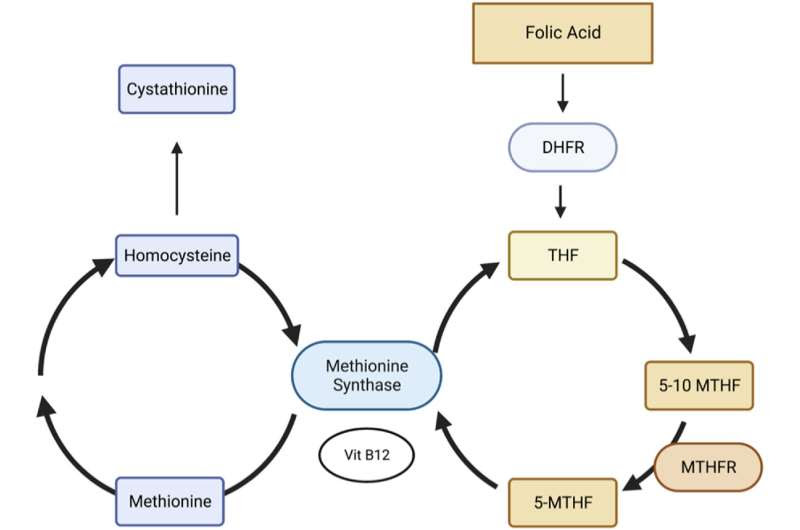
Metabolism of folate and folic acid through the one-carbon pathway. DHFR: dihydrofolate reductase; THF: tetrahydrofolate; 5–10 MTHF: 5–10 methylene tetrahydrofolate; MTHFR: methylenetetrahydrofolate reductase; 5-MTHF: 5-methyl tetrahydrofolate; Vit B12: Vitamin B12, a cofactor for methionine synthase. Credit: Heliyon (2023). DOI: 10.1016/j.heliyon.2023.e15387
Tulane University researchers have discovered a possible genetic cause for hypermobility (commonly known as double-jointedness) and a range of associated connective tissue disorders such as hypermobile Ehlers-Danlos syndrome, according to preliminary findings published in the journal Heliyon.
You may know someone with overly flexible joints, a friend or family member who can easily slide into a split or bend limbs to impossible angles. But hypermobility is a more serious condition than being “double-jointed.”
For those with hypermobile Ehlers-Danlos syndrome (EDS), the same conditions that create fragile connective tissue can cause a range of symptoms that, on the surface, can seem unrelated: physical conditions such as joint pain, chronic fatigue, thin tooth enamel, dizziness, digestive trouble and migraines; and psychiatric disorders, such as anxiety and depression. Women with hypermobile EDS may also be at increased risk for endometriosis or uterine fibroids.
Researchers have long struggled to find the cause of hypermobility and hypermobile EDS. Of the 13 subtypes of EDS, hypermobile EDS comprises more than 90% of the cases. But until this study, hypermobile EDS was the only subtype without a known genetic correlate. As a result, symptoms have often been treated individually rather than as the result of a single cause.
Researchers at Tulane University School of Medicine have linked hypermobility to a deficiency of folate—the natural form of vitamin B9—caused by a variation of the MTHFR gene.
“You’ve got millions of people that likely have this, and until now, there’s been no known cause we’ve known to treat,” said Dr. Gregory Bix, director of the Tulane University Clinical Neuroscience Research Center. “It’s a big deal.”
Those with this genetic variant can’t metabolize folate, which causes unmetabolized folate to accumulate in the bloodstream. The folate deficiency may prevent key proteins from binding collagen to the extracellular matrix. This results in more elastic connective tissue, hypermobility, and a potential cascade of associated conditions.
The discovery could help doctors more accurately diagnose hypermobility and hypermobile EDS by looking for elevated folate levels in blood tests as well as the MTHFR genetic variant.
“Hypermobility is widespread and unfortunately under-recognized,” said Dr. Jacques Courseault, medical director of the Tulane Fascia Institute and Treatment Center. “I’m excited about being able to treat the masses where people aren’t going their whole lives being frustrated and not getting the treatment they need.”
Previously, hypermobility could only be diagnosed by the Beighton score, a somewhat controversial physical exam that involves measuring the bend of the spine, fingers and limbs. Combined with a historic lack of acceptance of hypermobility as a distinct body type that requires specialized treatment, the number of people with hypermobility is unclear, though it could comprise more than half the world’s population.
“Hypermobility is not rare,” Courseault said. “Hypermobility is like a Ferrari that requires a lot of maintenance and the best synthetic oil. After knowing a patient’s name and date of birth, I think it’s prudent for clinicians to know which of these body types they have.”
Doctors discovered the connection between folate deficiency and the MTHFR gene by working with patients at Tulane’s Hypermobility and Ehlers-Danlos Clinic, the only such clinic in the U.S. that focuses on fascia disorders. Blood tests of hypermobile patients who showed signs of associated medical conditions revealed elevated levels of unmetabolized folate. Subsequent tests showed that most of those with elevated folate serum levels had the genetic polymorphism.
The good news is a treatment already exists. Methylated folate—folate that is already processed—is FDA-approved and widely available.
“It’s an innocuous treatment,” Bix said. “It’s not dangerous, and it’s a vitamin that can improve people’s lives. That’s the biggest thing: We know what’s going on here, and we can treat it.”
Though Courseault said more lab research and clinical testing needs to be done, patients who have been treated with folate have shown improvement: less pain, less brain fog, fewer allergies and improved gastrointestinal function.
“We’ve discovered something in medicine that can help, not a small group of people, but potentially many across the world,” Courseault said. “This is real, it’s been vetted out well and clinically we’re noticing a difference.”

Leave a Reply