Christine Kilgore
July 09, 2024
WASHINGTON, DC — The chronic, excessive fragility of aging and sun-damaged skin has a name in the medical literature: dermatoporosis. This identification is helpful because it validates patients’ suffering and conveys the skin’s vulnerability to serious medical complications, said Adam Friedman, MD, at the ElderDerm conference on dermatology in the older patient.

Adam Friedman, MD
Key features of dermatoporosis include atrophic skin, solar purpura, white pseudoscars, easily acquired skin lacerations and tears, bruises, and delayed healing. “We’re going to see more of this, and it will more and more be a chief complaint of patients,” said Friedman, professor and chair of dermatology at George Washington University (GWU) in Washington, DC, and co-chair of the meeting. GWU hosted the conference, describing it as a first-of-its-kind meeting dedicated to improving dermatologic care for older adults.
Dermatoporosis was described in the literature in 2007 by dermatologists at the University of Geneva in Switzerland. “It is not only a cosmetic problem,” Friedman said. “This is a medical problem…which can absolutely lead to comorbidities [such as deep dissecting hematomas] that are a huge strain on the healthcare system.”
Dermatologists can meet the moment with holistic, creative combination treatment and counseling approaches aimed at improving the mechanical strength of skin and preventing potential complications in older patients, Friedman said at the meeting.
He described the case of a 76-year-old woman who presented with dermatoporosis on her arms involving pronounced skin atrophy, solar purpura, and a small covered laceration. “This was a patient who was both devastated by the appearance” and impacted by the pain and burden of dressing frequent wounds, said Friedman, who is also the director of the Residency Program, of Translational Research, and of Supportive Oncodermatology, all within the Department of Dermatology at GWU.
With 11 months of topical treatment that included daily application of calcipotriene 0.05% ointment and nightly application of tazarotene 0.045% lotion and oral supplementation with 1000-mg vitamin C twice daily and 1000-mg citrus bioflavonoid complex daily, as well as no changes to the medications she took for various comorbidities, the solar purpura improved significantly and “we made a huge difference in the integrity of her skin,” he said.
Friedman also described this case in a recently published article in the Journal of Drugs in Dermatology titled “What’s Old Is New: An Emerging Focus on Dermatoporosis.”
Likely Pathophysiology
Advancing age and chronic ultraviolet (UV) radiation exposure are the chief drivers of dermatoporosis. In addition to UVA and UVB light, other secondary drivers include genetic susceptibility, topical and systematic corticosteroid use, and anticoagulant treatment.
Its pathogenesis is not well described in the literature but is easy to envision, Friedman said. For one, both advancing age and exposure to UV light lead to a reduction in hygroscopic glycosaminoglycans, including hyaluronate (HA), and the impact of this diminishment is believed to go “beyond [the loss of] buoyancy,” he noted. Researchers have “been showing these are not just water-loving molecules, they also have some biologic properties” relating to keratinocyte production and epidermal turnover that appear to be intricately linked to the pathogenesis of dermatoporosis.
HAs have been shown to interact with the cell surface receptor CD44 to stimulate keratinocyte proliferation, and low levels of CD44 have been reported in skin with dermatoporosis compared with a younger control population. (A newly characterized organelle, the hyaluronosome, serves as an HA factory and contains CD44 and heparin-binding epidermal growth factor, Friedman noted. Inadequate functioning may be involved in skin atrophy.)
Advancing age also brings an increase in matrix metalloproteinases (MMPs)–1, –2, and –3, which are “the demolition workers of the skin,” and downregulation of a tissue inhibitor of MMPs, he said.
Adding insult to injury, dermis-penetrating UVA also activates MMPs, “obliterating collagen and elastin.” UVB generates DNA photoproducts, including oxidative stress and damaging skin cell DNA. “That UV light induces breakdown [of the skin] through different mechanisms and inhibits buildup is a simple concept I think our patients can understand,” Friedman said.
Multifaceted Treatment
For an older adult, “there is never a wrong time to start sun-protective measures” to prevent or try to halt the progression of dermatoporosis, Friedman said, noting that “UV radiation is an immunosuppressant, so there are many good reasons to start” if the adult is not already taking measures on a regular basis.
Potential treatments for the syndrome of dermatoporosis are backed by few clinical studies, but dermatologists are skilled at translating the use of products from one disease state to another based on understandings of pathophysiology and mechanistic pathways, Friedman commented in an interview after the meeting.
For instance, “from decades of research, we know what retinoids will do to the skin,” he said in the interview. “We know they will turn on collagen-1 and -3 genes in the skin, and that they will increase the production of glycosaminoglycans…By understanding the biology, we can translate this to dermatoporosis.” These changes were demonstrated, for instance, in a small study of topical retinol in older adults.
Studies of topical alpha hydroxy acid (AHA), moreover, have demonstrated epidermal thickening and firmness, and “some studies show they can limit steroid-induced atrophy,” Friedman said at the meeting. “And things like lactic acid and urea are super accessible.”
Topical dehydroepiandrosterone is backed by even less data than retinoids or AHAs are, “but it’s still something to consider” as part of a multimechanistic approach to dermatoporosis, Friedman shared, noting that a small study demonstrated beneficial effects on epidermal atrophy in aging skin.
The use of vitamin D analogues such as calcipotriene, which is approved for the treatment of psoriasis, may also be promising. “One concept is that [vitamin D analogues] increase calcium concentrations in the epidermis, and calcium is so central to keratinocyte differentiation” and epidermal function that calcipotriene in combination with topical steroid therapy has been shown to limit skin atrophy, Friedman noted.
Nutritionally, low protein intake is a known problem in the older population and is associated with increased skin fragility and poorer healing. From a prevention and treatment standpoint, therefore, patients can be counseled to be attentive to their diets, Friedman said. Experts have recommended a higher protein intake for older adults than for younger adults; in 2013, an international group recommended a protein intake of 1-1.5 g/kg/d for healthy older adults and more for those with acute or chronic illness.
“Patients love talking about diet and skin disease…and they love over-the-counter nutraceuticals as well because they want something natural,” Friedman said. “I like using bioflavonoids in combination with vitamin C, which can be effective especially for solar purpura.”
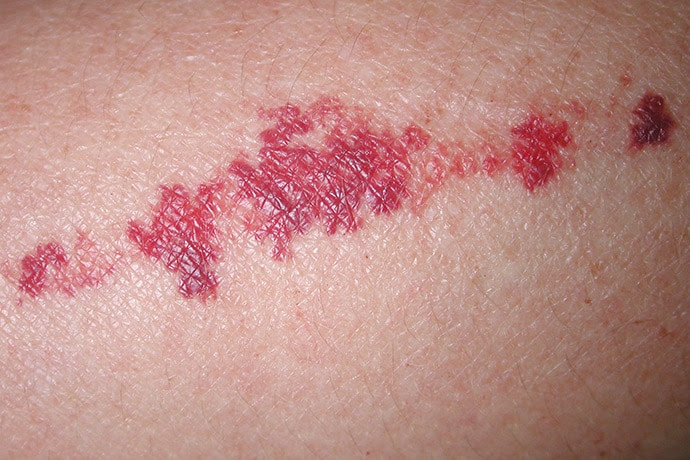
Actinic senile purpura, pictured here, is a common feature of dermatoporosis.
A 6-week randomized, placebo-controlled, double-blind trial involving 67 patients with purpura associated with aging found a 50% reduction in purpura lesions among those took a particular citrus bioflavonoid blend twice daily. “I thought this was a pretty well-done study,” he said, noting that patient self-assessment and investigator global assessment were utilized.
Skin Injury and Wound Prevention
In addition to recommending gentle skin cleansers and daily moisturizing, dermatologists should talk to their older patients with dermatoporosis about their home environments. “What is it like? Is there furniture with sharp edges?” Friedman advised. If so, could they use sleeves or protectors on their arms or legs “to protect against injury?”
In a later meeting session about lower-extremity wounds on geriatric patients, Michael Stempel, DPM, assistant professor of medicine and surgery and chief of podiatry at GWU, said that he was happy to hear the term dermatoporosis being used because like diabetes, it’s a risk factor for developing lower-extremity wounds and poor wound healing.
He shared the case of an older woman with dermatoporosis who “tripped and skinned her knee against a step and then self-treated it for over a month by pouring hydrogen peroxide over it and letting air get to it.” The wound developed into “full-thickness tissue loss,” said Stempel, also medical director of the Wound Healing and Limb Preservation Center at GWU Hospital.
Misperceptions are common among older patients about how a simple wound should be managed; for instance, the adage “just let it get air” is not uncommon. This makes anticipatory guidance about basic wound care — such as the importance of a moist and occlusive environment and the safe use of hydrogen peroxide — especially important for patients with dermatoporosis, Friedman commented after the meeting.
Dermatoporosis is quantifiable, Friedman shared during the meeting, with a scoring system having been developed by the researchers in Switzerland who originally coined the term. Its use in practice is unnecessary, but its existence is “nice to share with patients who feel bothered because oftentimes, patients feel it’s been dismissed by other providers,” Friedman said. “Telling your patients there’s an actual name for their problem, and that there are ways to quantify and measure changes over time, is validating.”
Its recognition as a medical condition, Friedman added, also enables the dermatologist to bring it up and counsel appropriately — without a patient feeling shame — when it is identified in the context of a skin excision, treatment of a primary inflammatory skin disease, or management of another dermatologic problem.
Friedman disclosed that he is a consultant/advisory board member for L’Oréal, La Roche-Posay, Galderma, and other companies; a speaker for Regeneron/Sanofi, Incyte, BMD, and Janssen; and has grants from Pfizer, Lilly, Incyte, and other companies. Stempel reported no disclosures.

Leave a Reply