August 8, 2024
by Taylor Barnes, Baylor College of Medicine
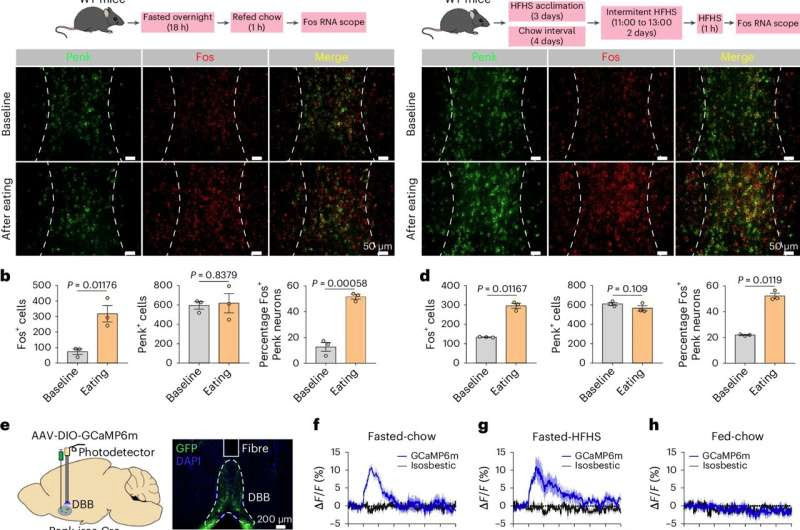
DBBPenk neurons are activated by food. Credit: Nature Metabolism (2024). DOI: 10.1038/s42255-024-01099-4
People eat either because they are hungry or for pleasure, even in the absence of hunger. While hunger-driven eating is fundamental for survival, pleasure-driven feeding may accelerate the onset of obesity and associated metabolic disorders.
A study published in Nature Metabolism reveals neural circuits in the mouse brain that promote hunger-driven feeding and suppress pleasure-driven eating. The findings open new possibilities for developing strategies to combat obesity.
“Ideal feeding habits would balance eating for necessity and for pleasure, minimizing the latter,” said co-corresponding author Dr. Yong Xu, professor of pediatrics—nutrition and associate director for basic sciences at the USDA/ARS Children’s Nutrition Research Center at Baylor College of Medicine. “In this study we identified a group of neurons that regulates balanced feeding in the brain.”
Previous studies have highlighted the role of neurons identified by the GABAergic proenkephalin (Penk) marker–an endogenous opioid hormone–in feeding and body weight balance. However, their contribution to regulating hunger- and pleasure-driven feeding had not been elucidated.
In this study, Xu and his colleagues showed that activation of Penk neurons in the brain region called diagonal band of Broca (DBB) of male mice supports an ideal feeding pattern, increasing hunger-driven feeding while reducing pleasure-driven eating.
“I was surprised by this finding,” Xu said. “We and other groups had previously shown that certain groups of neurons affect both feeding types in the same way—they either increase or decrease both types. Here we found that activating DBB-Penk neurons has opposite effects in the two types of feeding. They increase hunger-driven feeding while decreasing eating for pleasure.”
The researchers investigated the mechanism mediating these opposite effects. They discovered that DBB-Penk neurons project into two different brain areas, one regulates hunger-driven feeding and the other controls pleasure-driven eating.
“A subset of DBB-Penk neurons that projects to the paraventricular nucleus of the hypothalamus is preferentially activated upon food presentation during fasting periods, facilitating hunger-driven feeding,” Xu said. “On the other hand, a separate subset of DBB-Penk neurons that projects to a different brain region, the lateral hypothalamus, is preferentially activated when detecting high-fat, high-sugar (HFHS) foods and inhibits their consumption. This is the first study to show a neural circuit that is activated by a reward, HFHS, but leads to terminating instead of continuing the pleasurable activity.”
Strikingly, mice in which the entire DBB-Penk population had been eliminated, when given free choice of chow and HFHS diets, reduced consumption of chow but increased intake of the HFHS diet, resulting in accelerated development of obesity and metabolic disturbances.
“Our findings indicate that the development of obesity is associated with impaired function of some of these brain circuits in mice,” Xu said. “We are interested in further investigating molecular markers within the circuits that could be suitable targets for treatment of human diseases such as obesity.”
More information: Hailan Liu et al, Distinct basal forebrain-originated neural circuits promote homoeostatic feeding and suppress hedonic feeding in male mice, Nature Metabolism (2024). DOI: 10.1038/s42255-024-01099-4
Journal information: Nature Metabolism
Provided by Baylor College of Medicine

Leave a Reply