JULY 23, 2024
by NIH/National Institute of Allergy and Infectious Diseases
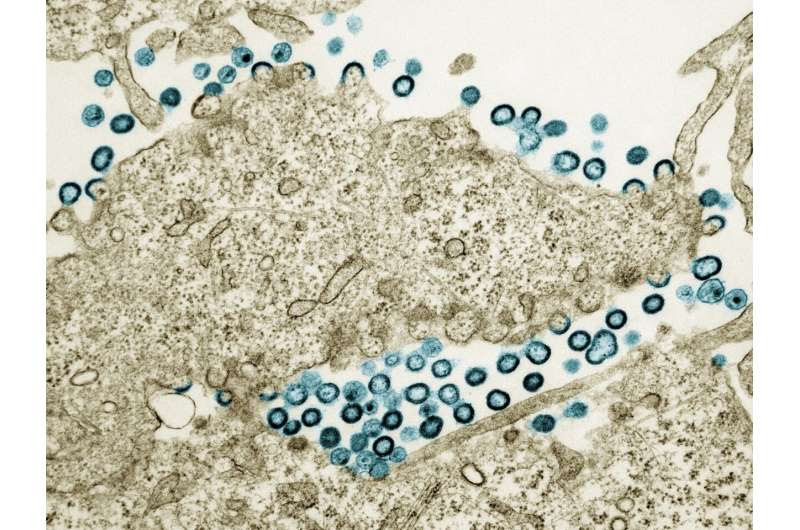
Transmission electron micrograph of HIV-1 virus particles (teal) budding and replicating from a segment of a chronically infected H9 cell (tan). Particles are in various stages of maturity; arc/semi-circles are immature particles that have started to form but are still part of the cell. Immature particles slowly change morphology into mature forms and exhibit the classic “conical or spherical-shaped core.” Image captured at the NIAID Integrated Research Facility in Fort Detrick, Maryland. Credit: NIAID
Current or previous use of the antiretroviral drug (ARV) abacavir was associated with an elevated risk of major adverse cardiovascular events (MACE) in people with HIV, according to an exploratory analysis from a large international clinical trial. There was no elevated MACE risk for the other antiretroviral drugs included in the analysis. The findings will be presented at the 2024 International AIDS Conference (AIDS 2024) in Munich, Germany.
The Randomized Trial to Prevent Vascular Events in HIV (REPRIEVE) enrolled 7,769 study participants with HIV from 12 countries that had found that daily use of a cholesterol-fighting statin drug reduced the risk of major adverse cardiovascular events, such as heart attacks and strokes, by more than one-third. The REPRIEVE study team also performed statistical analyses to assess whether select ARVs were associated with MACE risk among study participants, all of whom had low-to-moderate cardiovascular disease risk. The ARVs selected for analysis had previously been linked to cardiovascular risk and included abacavir, tenofovir, zidovudine, stavudine, and drugs from a class called protease inhibitors (PIs). All were taken as part of multi-drug ART regimens.
Overall, 22% of study participants reported prior exposure to abacavir, 86% to tenofovir, 49% to zidovudine or stavudine, and 47% to PIs. At study entry, 13% of participants were taking abacavir, 61% were taking tenofovir, 10% were taking zidovudine or stavudine, and 26% were taking PIs. In the investigators’ analyses, participants with prior and current use of abacavir had a 50% and 42% elevated risk of MACE, respectively, compared to participants with no abacavir exposure. Former or current use of other ARVs was not associated with any change in MACE risk, and the co-administration of common ARV drug classes as part of an ART regimen did not impact the elevated MACE risk among participants with current or prior abacavir exposure.
According to the authors, these findings align with previous studies that also identified an elevated cardiovascular disease risk associated with abacavir. They suggest that more research is needed to better understand the increased risk observed in this analysis, including how these findings should be considered in the context of known cardiovascular disease risk factors, such as dyslipidemia, diabetes and hypertension, for people with HIV.
More information: CJ Fichtenbaum et al, Abacavir is associated with elevated risk for cardiovascular events in the REPRIEVE trial. International AIDS Conference. Friday, July 26, 2024
Provided by NIH/National Institute of Allergy and Infectious Diseases

Leave a Reply