The FDA approved a new medicine Friday to reduce the complications associated with sickle cell disease, a rare blood disorder.
The drug, Endari, is made by privately held Emmaus Medical and is the first new treatment for sickle cell disease to secure FDA approval in almost 20 years. However, the active ingredient in Endari—L-glutamine—is an old chemical that can be purchased over the counter, which could complicate Emmaus’s ability to obtain insurance coverage.
In Emmaus’s pivotal clinical trial, treatment with Endari over 48 weeks reduced the frequency and length of hospital visits for sickle cell pain crises compared to placebo. Commonly reported side effects of the drug included constipation, nausea, and headache.
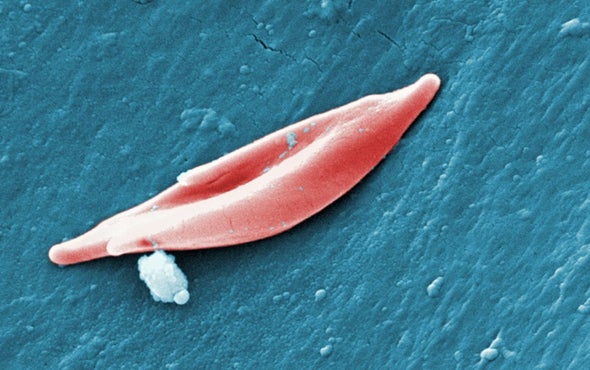
Sickle cell disease is a rare, inherited disorder characterized by abnormally sickle-shaped red blood cells. These malformed red blood cells clog blood vessels and cut off oxygen to the body’s tissues, leading to episodes of severe pain and organ damage.
Approximately 100,000 people in the U.S. have sickle cell disease, according to the National Institutes of Health.
While Endari focuses on reducing the complications of sickle cell disease, Bluebird Bio is developing a gene therapy to treat the disease’s underlying cause and potentially cure patients. Research using the CRISPR gene-editing technology aims to accomplish the same goal.
Other companies working on new, novel drugs targeting sickle cell include Global Blood Therapeutics and Novartis.
