by Kurt Bodenmüller, University of Zurich
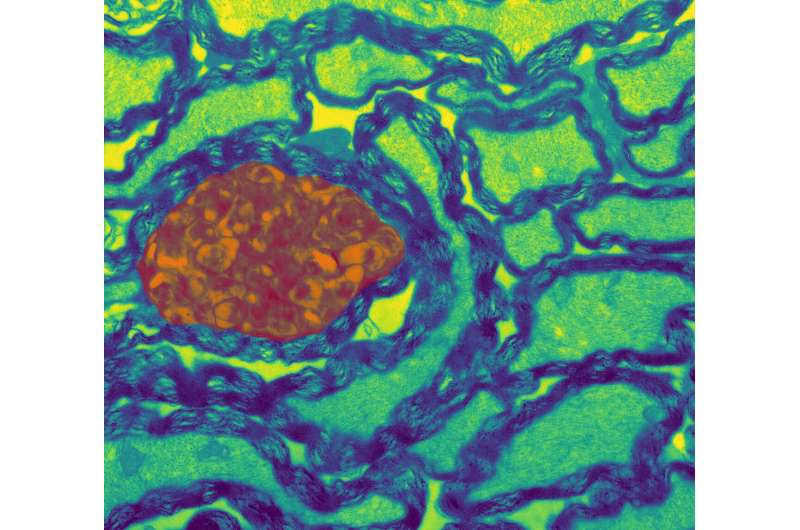
Cross section of nerve fibers in the mouse optic nerve. Credit: Zoe Looser and Aiman Saab, University of Zurich
Brain function depends on the swift movement of electrical signals along axons, the long extensions of nerve cells that connect billions of brain cells. The nerve fibers are insulated by a fatty layer called myelin, which is produced by specialized cells called oligodendrocytes. These cells wrap around and insulate nerve fibers ensuring the rapid and efficient transmission of signals that are essential for brain function.
Now, a team of neuroscientists led by Aiman Saab at the Institute of Pharmacology and Toxicology at the University of Zurich (UZH) has discovered a new central function of these myelin-forming cells in the mouse brain.
“We found that oligodendrocytes not only detect the signals from active nerve fibers, but also respond to them by immediately accelerating their consumption of glucose, a primary energy source,” says Saab. In this way, the oligodendrocytes deliver energy-rich molecules to the rapidly firing axons to support their dynamic energy needs.
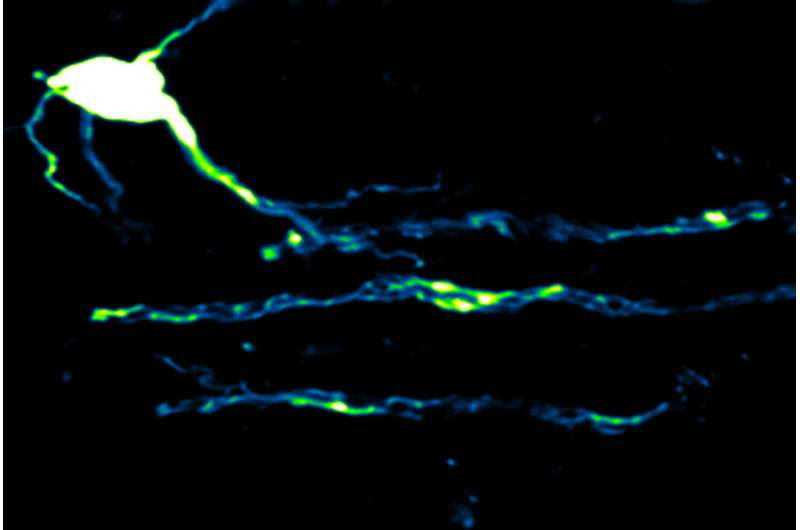
These findings are published in the journal Nature Neuroscience.
Firing nerve fibers in the brain are supplied with energy on demandSingle oligodendrocyte. Credit: Zoe Looser and Aiman Saab, University of Zurich
Potassium is key signal that activates oligodendrocytes
To understand how electrically active axons communicate with their surrounding oligodendrocytes, the researchers studied the mouse optic nerve, an ideal pathway for stimulating and monitoring the electrical activity of myelinated axons. To trigger axonal firing and to observe how oligodendrocytes respond to this activity, they used tiny biosensors: Engineered proteins that serve as microscopic detectors of molecular changes.
“Using a variety of chemicals and inhibitors, we were able to show that potassium, released by axons during firing, is the key signal that activates the oligodendrocytes,” says Zoe Looser, the first author of the study.
Missing potassium channels lead to nerve fiber damage
The researchers also identified a specific potassium channel, called Kir4.1, as a key player in the communication between nerve fibers and oligodendrocytes. To study its role, the team used genetically modified mice that lacked these channels in their oligodendrocytes.
In these mice, axons surrounded by oligodendrocytes without these potassium channels showed reduced lactate levels, a key byproduct of glucose metabolism, and a diminished response in lactate surge upon activation. “These changes were associated with reduced glucose metabolism in nerve fibers and ultimately led to severe axon damage as the mice aged,” adds Looser.
The findings underscore the essential role of oligodendrocytes in regulating the metabolic processes within axons that are vital for sustaining healthy brain connections. Glucose not only fuels nerve fibers but also supports protective mechanisms against oxidative stress.
“Disruptions in axonal glucose metabolism due to oligodendrocyte dysfunction could lead to nerve damage, which is a concern in aging and several neurodegenerative diseases, such as multiple sclerosis and Alzheimer’s disease,” says Aiman Saab.
The next step is to understand how the regulation of glucose by oligodendrocytes affects specific functions of the nerve fibers, with a particular focus on their health during aging and neurological disorders.
More information: Zoe J. Looser et al, Oligodendrocyte–axon metabolic coupling is mediated by extracellular K+ and maintains axonal health, Nature Neuroscience (2024). DOI: 10.1038/s41593-023-01558-3
Provided by University of Zurich

Leave a Reply