Super-precise gene-editing approach switches off a gene in the liver that regulates ‘bad’ cholesterol.
Miryam Naddaf
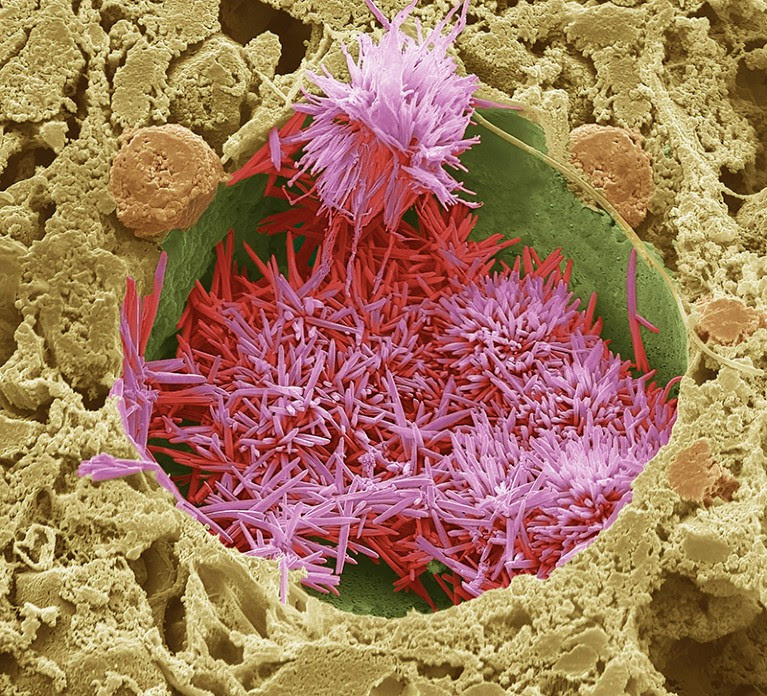
Cholesterol crystals (red) in a lipid droplet in a human liver.Credit: Steve Gschmeissner/Science Photo Library
The first trial in humans of the precise gene-editing technique known as base editing has shown promising results for keeping cholesterol levels in check.
The approach injects into people a treatment called VERVE-101, which permanently deactivates a gene in the liver called PCSK9. That gene controls the level of low-density lipoprotein (LDL), or ‘bad’ cholesterol — a key contributor to heart disease.
Verve Therapeutics, the biotechnology firm in Boston, Massachusetts, behind the treatment, reported that a one-time injection of VERVE-101 reduced the amount of LDL in the blood by up to 55% in its trial participants, who had a condition that causes lifelong high LDL.

Super-precise CRISPR tool enters US clinical trials for the first time
“It’s a tremendous scientific milestone because it’s the first time that they’ve been able to show that a single base pair of DNA editing, using CRISPR technology in humans, has had a clinical effect,” says Ritu Thamman, a cardiologist at the University of Pittsburgh in Pennsylvania. “From the clinical point of view, it has the potential to open a new way of treating coronary artery disease” that could involve people receiving a ‘one and done’ treatment rather than taking daily pills.
But the findings have also drawn criticism. Two serious adverse events in the trial, including a death, have raised safety concerns, and Verve’s share price plummeted by nearly 40% following the results’ release despite their promise.
Verve reported the findings, interim results from a phase 1b trial conducted in the United Kingdom and New Zealand, at a meeting of the American Heart Association in Philadelphia on 12 November. It will continue its trial next year in the United States, after receiving approval last month from the US Food and Drug Administration to enroll participants there.
Precise edits
Base editing uses CRISPR-Cas9 machinery to make very precise edits to a gene — chemically changing single nucleotide bases — without breaking the double strands of DNA as other gene-editing approaches do. The technique was developed by a team led by chemical biologist David Liu at Harvard University in Cambridge, Massachusetts, in 2018.
Super-precise new CRISPR tool could tackle a plethora of genetic diseases
By permanently switching off the PCSK9, VERVE-101 affects the enzymes encoded by the gene. These enzymes ordinarily prompt receptors for LDL-cholesterol, which are located on cell surfaces, to move inside the cell. With fewer available receptors to bind LDL, its levels in the blood increase. But when PCSK9 is deactivated, the enzyme loses function, reducing LDL levels.
The treatment aims to protect against heart attacks and strokes. “If the blood LDL-cholesterol is very low lifelong, it’s very hard to get a heart attack,” says Sekar Kathiresan, Verve’s co-founder and chief executive officer.
VERVE-101 consists of two RNA molecules packaged in a lipid nanoparticle — an mRNA molecule that edits adenine bases in DNA and a ‘guide RNA’ molecule to recognize PCSK9. After the treatment is injected, liver cells take up these nanoparticles, and once inside cells they make their way into the nucleii. Then, the base editor makes a single-letter change to the PCSK9 gene sequence, swapping an adenosine base with a guanine base. This turns off the gene and prevents liver cells from producing PCSK9 proteins.
Dosing strategy
Verve trialled the treatment in 10 people who had a life-threatening inherited disease called heterozygous familial hypercholesterolemia (HeFH), which causes high LDL levels from birth. The condition, which affects more than three million people in the United States and Europe, can cause premature heart attacks, sometimes in childhood. The participants also suffered from severe advanced coronary disease and took maximum doses of lipid-lowering tablets such as statins.
CRISPR treatment inserted directly into the body for first time
Before receiving VERVE-101, the study participants had an average LDL level of 193 mg/dL. After 28 days, participants treated with either a high or low dose of VERVE-101 had their PCSK9 levels reduced by up to 84%. Their LDL-cholesterol levels dropped by up to 55% .
That drop is large compared with conventional treatments. “We don’t see that with the statin — we never see that much of a difference,” says Thamman.
The 55% reduction of LDL persisted for 6 months in participants who received the higher dose of VERVE-101. In a preclinical study with monkeys, LDL-cholesterol reduction lasted 2.5 years after a single dose of the treatment.
“We learned that we get durable LDL lowering with the gene-editing strategy. This has never been done before,” said Karol Watson, a cardiologist at the University of California, Los Angeles, at a 12 November press briefing announcing the findings.
Safety concerns
The treatment came with some side effects: participants experienced brief flu-like symptoms, including fever, headaches and body aches, as well as a temporary increase in liver enzymes, which returned to normal within days.
But two more serious events have raised some concerns. Two out of the ten participants experienced cardiovascular events, including one who died from a heart attack five weeks after receiving VERVE-101 another who had a heart attack after one day. An independent safety board concluded that the first event was expected in people who had such advanced heart disease and was not related to treatment. The board recommended the trial’s continuation of trial enrolment without changes to the drug protocol.
Super-precise CRISPR tool enhanced by enzyme engineering
Some analysts blamed the sharp drop in Verve’s share price on the safety concerns, and say that potentially risky gene therapies are a hard sell for diseases that have working conventional treatments. Gene therapies should be prioritized for conditions that have no available treatments, say some researchers.
“The sentiment for editing when there are viable alternatives is going to be a challenge. Time will tell if non-rare is viable,” Michael Torres, cancer biologist and co-founder of genetic-medicines company ReCode Therapeutics, wrote in a post on X, formerly Twitter.
“From a scientific standpoint, there is still a lot of going in terms of addressing some of the key aspects of this technology,” says Luigi Naldini, a gene therapist at the Vita-Salute San Raffaele University in Milan, Italy. “The delivery by nanoparticles is still in early stages in terms of tolerability.”
Targeted changes
There are other unknowns about the long-term effects of these genetic changes, says Naldini.
Gene-editing approaches carry the risk of ‘off target’ edits elsewhere in the genome. In animal studies, the Verve team found no off-target editing in mice, and no evidence of the changes in the PSCK9 gene becoming heritable in monkeys. Verve aims to select the best therapeutic dose from the trial next year and to launch a phase 2 trial in 2025.
The firm must also follow trial participants for 14 years, as mandated by the FDA for gene-editing therapies. “This is a gene editing study — you are changing the genome forever. Safety is going to be of the utmost importance, especially because there are currently safe and efficacious strategies available for lipid lowering,” said Watson at the press briefing.
doi: https://doi.org/10.1038/d41586-023-03543-z

Leave a Reply