by Case Western Reserve University
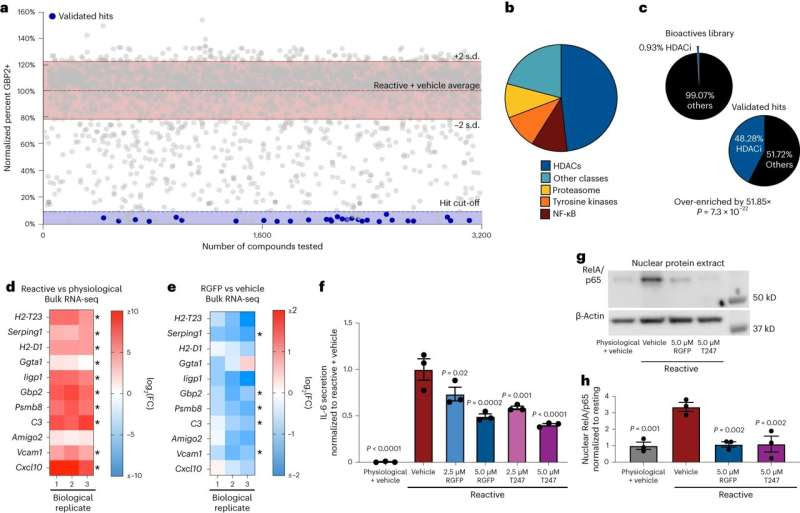
Phenotypic screen identifies HDAC3 as a regulator of pathological reactive astrocytes. a, Scatter plot of primary screen results displayed as percent GBP2 positive, normalized to reactive astrocyte plus vehicle controls for all nontoxic chemicals. Validated hit chemicals colored in blue. The dashed blue line represents the hit cut-off at a ≥90% decrease in GBP2-positive astrocytes compared to reactive astrocyte plus vehicle controls. The dashed red line represents the average percent GBP2 positive for reactive astrocytes plus vehicle set at 100%. Solid lines represent ±2 s.d. from the mean of reactive plus vehicle control wells. b, Pie chart depicting the chemical class breakdown of all 29 validated chemical hits. c, Pie charts depicting the frequency of HDAC inhibitor compounds enriched in the primary screen validated hit list compared to the primary screen chemical library as a whole, showing that HDAC inhibitors are significantly enriched in the validated hit list. P value generated by a two-tailed hypergeometric test. d,e, Heatmap of the log2(FC) from bulk RNA-seq analysis of reactive versus physiological (d) and RGFP966 (RGFP) versus vehicle (DMSO) treated reactive astrocytes (e). Red is upregulated and blue is downregulated. Data are presented as log2(FC) for n = 3 biological replicates with asterisks denoting a P < 0.05 calculated by DESeq2. f, Quantification of IL-6 ELISAs performed on astrocyte-conditioned media. Data presented as mean ± s.e.m. for n = 3 biological replicates with significance calculated compared to reactive plus vehicle control. P value generated by one-way ANOVA with Dunnett multiple comparison correction. g, Representative western blot image of nuclear protein extracts from physiological astrocytes, reactive astrocytes and reactive astrocytes treated with either of the HDAC3 inhibitors RGFP966 or T247 probed for RelA/p65 and β-actin. h, Quantification of experiments represented in g. Data are presented as mean ± s.e.m for n = 3 biological replicates (independent astrocyte isolations). P value generated by one-way ANOVA with Dunnett multiple comparison correction. Credit: Nature Neuroscience (2024). DOI: 10.1038/s41593-024-01580-z
A team led by scientists at the Case Western Reserve University School of Medicine has identified a new therapeutic approach for combating neurodegenerative diseases, offering hope of improved treatments for Alzheimer’s disease, Parkinson’s disease, Vanishing White Matter disease, and multiple sclerosis, among others.
Neurodegenerative diseases, which affect millions of people worldwide, occur when nerve cells in the brain or nervous system lose function over time and ultimately die, according to the National Institutes of Health. Alzheimer’s disease and Parkinson’s disease are the most common.
The research team’s new study, published in the journal Nature Neuroscience, focused on astrocytes—the brain’s most abundant cells, which normally support healthy brain function. Growing evidence indicates astrocytes can switch to a harmful state that increases nerve-cell loss in neurodegenerative diseases.
The researchers created a new cellular technique to test thousands of possible medications for their ability to prevent these rogue astrocytes from forming.
“By harnessing the power of high-throughput drug screening, we’ve identified a key protein regulator that, when inhibited, can prevent the formation of harmful astrocytes,” said Benjamin Clayton, lead author and National Multiple Sclerosis Society career transition fellow in the laboratory of Paul Tesar at the Case Western Reserve School of Medicine.
They found that blocking the activity of a particular protein called HDAC3 may prevent the development of dangerous astrocytes. The scientists discovered that by administering medications that specifically target HDAC3, they were able to prevent the development of dangerous astrocytes and significantly increase the survival of nerve cells in mouse models.
“This research establishes a platform for discovering therapies to control diseased astrocytes and highlights the therapeutic potential of regulating astrocyte states to treat neurodegenerative diseases,” said Tesar, the Dr. Donald and Ruth Weber Goodman Professor of Innovative Therapeutics and the study’s principal investigator.
Tesar, also director of the School of Medicine’s Institute for Glial Sciences, said more research needs to be done before patients might benefit from the promising approach. But, he said, their findings could lead to the creation of novel therapies that disarm harmful astrocytes and support neuroprotection—perhaps improving the lives of people with neurodegenerative illnesses in the future.
“Therapies for neurodegenerative disease typically target the nerve cells directly,” Tesar said, “but here we asked if fixing the damaging effects of astrocytes could provide therapeutic benefit. Our findings redefine the landscape of neurodegenerative disease treatment and open the door to a new era of astrocyte targeting medicines.”
More information: Benjamin L. L. Clayton et al, A phenotypic screening platform for identifying chemical modulators of astrocyte reactivity, Nature Neuroscience (2024). DOI: 10.1038/s41593-024-01580-z
Provided by Case Western Reserve University

Leave a Reply