HEALTH
15 May 2024
ByDAVID NIELD
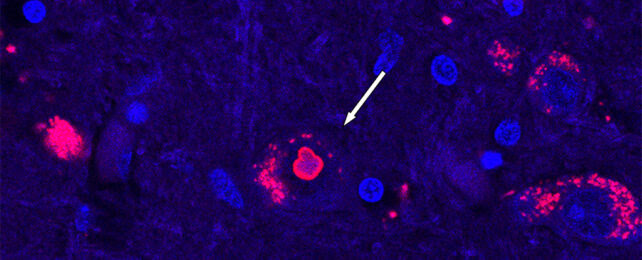
Brain tissue scan, with the bright red ring showing a protein clump that only shows up in patients with SCA4. (Mandi Gandelman)
You might not have heard of spinocerebellar ataxia type 4 (SCA4), as it’s an incredibly rare condition – but after a quarter of a century of looking, the genetic coding behind SCA4 has now been identified, potentially opening up new treatment options for this serious disease.
An international team of researchers behind the discovery says that it pinpoints the cause of the debilitating disease for the first time. Those with SCA4 gradually develop worsening problems with walking and balance, and there’s currently no cure.
As SCA4 is clearly passed from generation to generation, scientists knew genetics were involved, but it took the latest in genome sequencing techniques, and a search of 6,495 genome sequencing datasets, to reveal that a section of a gene called ZFHX3 is the culprit behind the disease.
The gene ZFHX3 was highlighted using long-read genome sequencing, which – as the name suggests – means longer DNA fragments can be identified. ZFHX3 is in an area of DNA with a lot of repeated segments, making it hard to analyze.
What’s more, the mutation itself is one of these repeating segments.
“This mutation is a toxic expanded repeat and we think that it actually jams up how a cell deals with unfolded or misfolded proteins,” says neurologist Stefan Pulst from the University of Utah.
Part of ZFHX3 is much longer than it should be in people who develop SCA4, the research team found, and cells with this extended version show signs of being unhealthy – specifically, they can’t do the essential job of recycling proteins as they should.
Further research showed that the ZFHX3 mutation jammed up the protein recycling machinery, potentially leading to nerve cells being poisoned, and causing the crippling symptoms that worsen as SCA4 takes hold.
The researchers are keen to acknowledge the help they’ve had from families with SCA4, without whom the new discovery wouldn’t have been possible. The team was able to trace the disease all the way back to Salt Lake Valley in the 1840s.
“I’ve been working on SCA4 directly since 2010 when the first family approached me, and once you go to their homes and get to know them, they’re no longer the number on the DNA vial,” says neurologist Karla Figueroa from the University of Utah.
“These are people you see every day… you can’t walk away. This is not just science. This is somebody’s life.”
All types of spinocerebellar ataxia affect around 150,000 people in the US (population 341.5 million), and the amount of people with the specific type 4 are considerably less than that, but the new research means people affected can be tested for the disease-causing gene.
Further down the line, treatments could be worked on, now we know what is triggering SCA4. What’s more, the researchers behind the new study think something similar could be happening with other spinocerebellar ataxia types too.
“The only step to really improve the life of patients with inherited disease is to find out what the primary cause is,” says Pulst. “We now can attack the effects of this mutation potentially at multiple levels.”
The research has been published in Nature Genetics.

Leave a Reply