by Tata Institute of Fundamental Research
Feeding and fasting cycles exert control over metabolism and energy utilization. Aberrations are known to cause metabolic diseases, liver dysfunctions and accelerated aging. Expression and activity of the anti-aging factor SIRT1 has long been known to be beneficial in mitigating diseases such as diabetes, cardiovascular dysfunctions, neurodegeneration, cancer and aging. Global efforts are underway to both uncover molecular mechanisms that affect feed-fast cycles and also to regulate the activity of the longevity factor SIRT1.
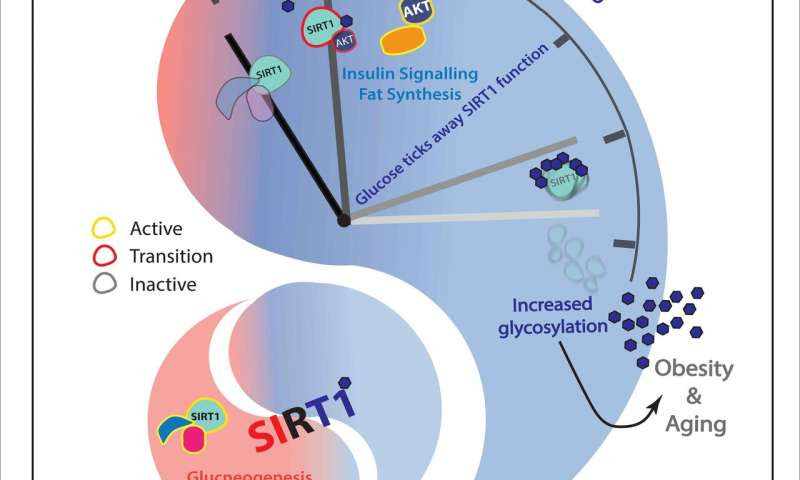
A novel discovery from TIFR shows that glucoseacts as a double-edged sword in regulating the functions of SIRT1. These results have been published in the journal PNAS. The study found that a glucose-derived cellular metabolite acted as a molecular switch to regulate both the extent and time of activity of the longevity factor, which affected gene expression and regulated metabolic flexibility in the liver. This study has immense therapeutic potential since the loss of SIRT1 is associated with obesity and aging, and its over-activation resulted in perturbed liver functions, inflammation and a pre-diabetic like state.
India has earned the dubious distinction of harbouring the world’s fastest-growing diabetic population, with an estimated 72 million cases in 2017, a figure expected to almost double by 2025. Increasing evidence indicates that feed-fast cycles are important for physiological homeostasis and aberrant feeding and/or fasting leads to metabolic diseases including non-alcoholic fatty liver disease (NAFLD), hyper-inflammation and aging. This has huge impact on public health and lifestyle-related disorders. The liver plays a pivotal role in maintaining organismal health and lifespan, and regulates both fat and glucose metabolism, ensuring appropriate utilization of energy sources during normal feed-fast cycles. Thus, identifying molecular mechanisms within liver cells that regulate the metabolic fitness of an organism becomes crucial.
The current study revealed that glucose controls the functions of SIRT1, whereby glucose itself binds and modifies SIRT1 and ultimately reduces its levels. While this reduction is required for metabolic oscillation during feed-fast cycles, sustained glycosylation as seen in obesity and aging abrogates the protective functions of SIRT1. Surprisingly, genetic tricks that eliminate this control and which result in overactivation of the longevity factor during feed-fast cycles was also detrimental to liver physiology and resulted in increased blood glucose levels, akin to a pre-diabetic like state. Thus, it is exciting that both excessive modification by glucose (as in the case of obesity and aging) and no modification (as in the case of fasting) are detrimental to organismal physiology. Therefore, this study describes glucose-derived modification of the longevity factor SIRT1, which keeps a check on its activity and functions, specifies downstream molecular signaling, and fine tunes gene expression in the liver.
This tuning is essential for metabolic flexibility in normal feed-fast cycles and during aging. Interestingly, this glucose-dependent modification regulated organismal glucose homeostasis via modulating insulin signaling, mitochondrial functions and fat metabolism. While there are efforts to find therapeutic activators for SIRT1, the study shows that both over-activation and under-activation of this longevity factor could lead to diseases. Hence, ways to regulate this modification might be beneficial in tackling lifestyle disorders and age-related diseases.
More information: Tandrika Chattopadhyay el al., “Spatiotemporal gating of SIRT1 functions by O-GlcNAcylation is essential for liver metabolic switching and prevents hyperglycemia,” PNAS (2020). www.pnas.org/cgi/doi/10.1073/pnas.1909943117
Journal information: Proceedings of the National Academy of Sciences
Provided by Tata Institute of Fundamental Research

Leave a Reply