By Chris Melore
Research led by Dr. Rene Frank, University of Leeds
Jul 11, 2024
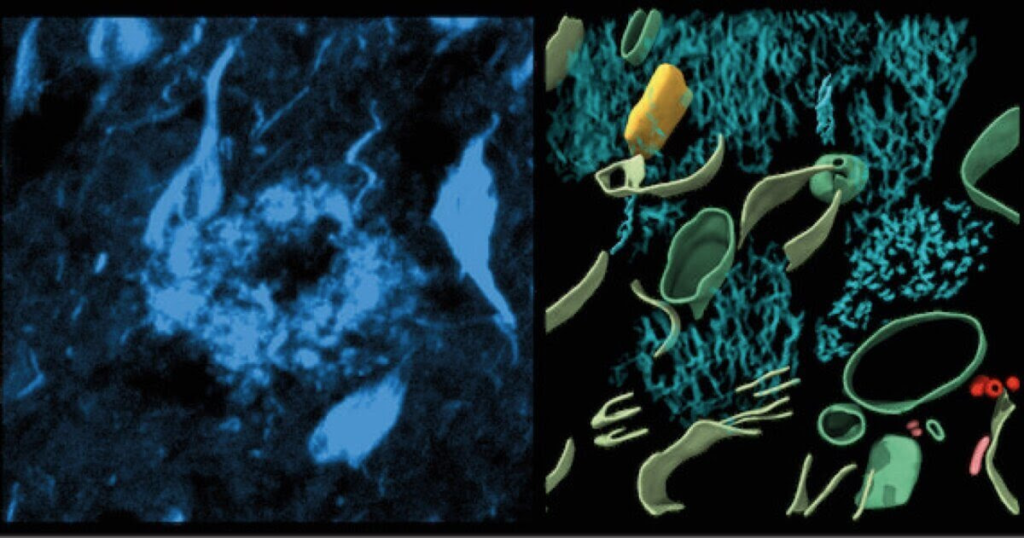
Left, fluorescence image of amyloid in cryo-preserved post-mortem human brain. Middle, 3-dimensional molecular architecture of β-amyloid plaque. Right, in-tissue structure of tau filaments within post-mortem brain. (CREDIT: University of Leeds)
LEEDS, United Kingdom — In a world-first achievement, scientists are giving us a peek into the human brain — revealing what exactly Alzheimer’s disease looks like at a molecular level. This breakthrough study, published in the journal Nature, could pave the way to better understand and potentially treat this devastating condition.
Imagine zooming in on a brain cell so closely that you can see individual proteins — the building blocks of life. That’s exactly what researchers at the University of Leeds and their collaborators have accomplished. They used cutting-edge technology to observe the tiniest structures within an Alzheimer’s patient’s brain, giving us an unprecedented look at the disease’s molecular landscape.
“This first glimpse of the structure of molecules inside the human brain offers further clues to what happens to proteins in Alzheimer’s disease but also sets out an experimental approach that can be applied to better understand a broad range of other devastating neurological diseases,” explains Dr. Rene Frank, the study’s lead author and an associate professor at the University of Leeds’s School of Biology, in a media release.
What exactly did scientists see?
The researchers focused on two proteins that play a starring role in Alzheimer’s: β-amyloid and tau. These troublemakers are well-known to scientists, but seeing them in their natural habitat in the brain is a game-changer.
β-amyloid forms sticky plaques outside brain cells, while tau creates abnormal filaments inside cells. Both of these protein problems are thought to interfere with how brain cells communicate, leading to memory loss, confusion, and brain cell death among Alzheimer’s patients.
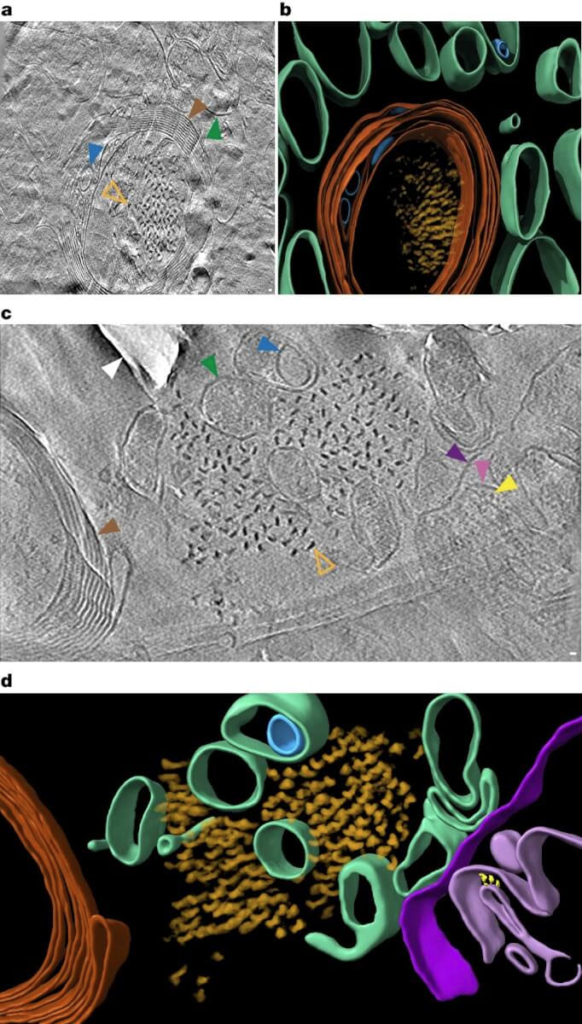
In situ cryoET of tau deposits in vitrified postmortem AD brain. (Credit: Nature)
The researchers used cryo-electron tomography guided by fluorescence microscopy to make this discovery. In simpler terms, it’s like a super-powerful 3D microscope that can work at extremely cold temperatures, preserving the delicate structures of brain tissue. This allowed the U.K. team to create detailed 3D maps of the brain at a molecular level. To put the scale into perspective, the proteins they observed are about a million times smaller than a grain of rice.
This study is part of a broader shift in how scientists approach structural biology. For decades, researchers have studied proteins in isolation, building up a vast catalog of molecular structures. However, proteins don’t exist in isolation in our bodies — they work together in complex networks.
By studying proteins directly within brain tissue, scientists can observe how these molecules interact and influence each other, especially in diseased states. This approach could lead to new targets for drugs and diagnostic tools, potentially revolutionizing how we approach Alzheimer’s and other neurological disorders.
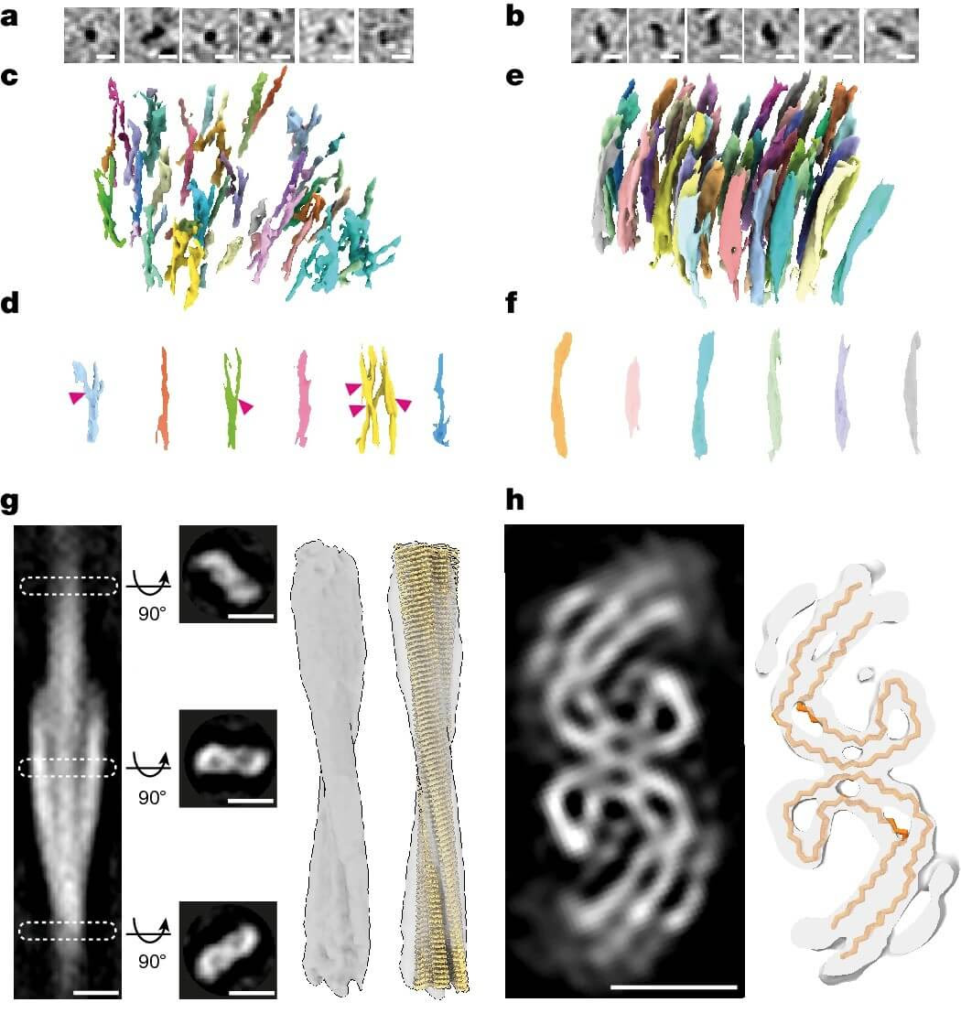
In-tissue architecture of β-amyloid fibrils and tau filaments, and subtomogram averaging of tau filaments within postmortem AD brain. (Credit: Nature)
The implications of this research extend far beyond Alzheimer’s research. The techniques developed here could be applied to study a wide range of brain diseases, offering new perspectives on conditions that have long puzzled medical science.
As the world population ages, understanding and treating dementia becomes increasingly crucial. In the United States, Alzheimer’s affects roughly seven million people, and that number may triple within the next 30 years. This research brings hope for better treatments and perhaps even prevention strategies in the future.
Paper Summary
Methodology
To achieve these groundbreaking results, the research team began by obtaining postmortem brain samples from Alzheimer’s patients and non-demented control subjects. These samples were rapidly frozen to preserve their native structures and then subjected to cryo-fluorescence microscopy (cryo-FM) to identify regions of interest containing amyloid and tau pathology.
Next, the samples were prepared for cryoET using cryo-focused ion beam (FIB) scanning electron microscopy (SEM). This step involved the careful lift-out of thin sections of brain tissue, which were then imaged using cryo-electron microscopy. The resulting tomographic volumes provided detailed three-dimensional views of the amyloid and tau structures within the brain tissue.
One of the key techniques used in this study was subtomogram averaging, which allowed the researchers to enhance the resolution of their images by averaging multiple tomograms. This approach enabled them to visualize the intricate details of amyloid fibrils and tau filaments, revealing their unique structural characteristics.
Key Results
The researchers found that Aβ plaques in Alzheimer’s disease brains contain a variety of structural elements, including fibrils, branched fibrils, and protofilament-like rods. These components were interlaced with non-amyloid constituents such as extracellular vesicles and cuboidal particles. The presence of these additional elements suggests a more complex architecture for amyloid plaques than previously thought.
In contrast, tau tangles were composed of unbranched filaments organized into parallel clusters. Subtomogram averaging of these filaments revealed detailed information about their polypeptide backbone conformation and filament polarity. Interestingly, the study found that tau filaments within a single cluster were similar to each other but differed between clusters, indicating spatial organization and heterogeneity of tau pathology within the brain.
Study Limitations
While this study’s findings are groundbreaking, the researchers also acknowledged several limitations. One of the primary challenges was the potential impact of postmortem interval and freeze-thaw steps on the integrity of the brain tissue. Although the team took measures to control for these variables, they cannot entirely rule out their influence on the observed structures.
Another limitation was the resolution of subtomogram averages for some tau filament clusters, which did not reach the level required to unambiguously determine specific tau amyloid folds. Additionally, the study was conducted on a limited number of brain samples, and further research is necessary to confirm these findings across a larger cohort of Alzheimer’s patients and control patients.
Discussion & Takeaways
By providing a detailed view of the in-situ structures of amyloid and tau proteins, this research enhances our understanding of the molecular mechanisms underlying Alzheimer’s disease. The discovery of the complex architecture of amyloid plaques and the spatial organization of tau filaments offers new avenues for exploring how these proteins contribute to neurodegeneration.
One of the most significant takeaways from this study is the potential for developing targeted therapies that address the specific structural characteristics of amyloid and tau aggregates. For example, treatments designed to disrupt the formation of branched amyloid fibrils or to prevent the clustering of tau filaments could prove more effective than current approaches.

SHARE:
About Chris Melore
Chris Melore has been a writer, researcher, editor, and producer in the New York-area since 2006. He won a local Emmy award for his work in sports television in 2011.

Leave a Reply