by Nagoya City University
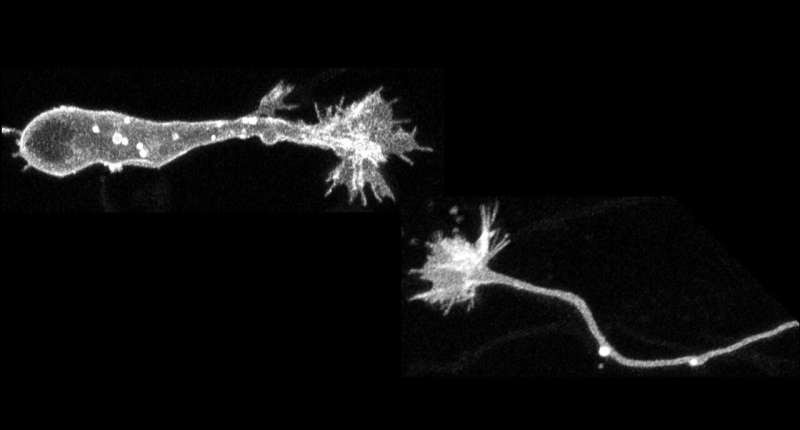
Collective time-lapse images of a migrating neuron (bottom) and an elongating axon (top) from super-resolution microscopy. Growth cones at the tips of the neurons are extended while the cell body migrates and the axon elongates. Credit: Nagoya City University Gradualte School of Medical Sciences
The structure and functions of the tip of migrating neurons remain elusive. A research group has found that the PTPσ-expressing growth cone senses the extracellular matrix and drives neuronal migration in the injured brain, leading to functional recovery.
The full findings of the study are published in a paper titled, “Identification of the growth cone as a probe and driver of neuronal migration in the injured brain,” in Nature Communications.
Neural stem cells are present in the postnatal mammalian brain and produce new neurons. New neurons migrate toward injured sites, and promoting neuronal migration results in functional recovery after brain injury. Nevertheless, there is an inhibitory effect on neuronal migration in the injured sites, the mechanisms of which need to be elucidated in order to improve recruitment of new neurons in the injured sites and thus to enhance the recovery after brain injury.
The migrating neurons possess an axonal growth cone-like structure at their tip, but the role of this structure in neuronal migration has not been fully understood.
The group, led by Kazunobu Sawamoto, Professor at Nagoya City University and National Institute for Physiological Sciences, and Chikako Nakajima and Masato Sawada, focused on elucidating the function of the growth cone-like structure of migrating neurons of the mouse brain. The researchers used super-resolution microscopy to study the cytoskeletal dynamics and molecular features of the neuronal tip.
They showed that the tip structure shares important functions with axonal growth cones. In short, the growth cone of cultured migrating neurons is responsive to external signals through tyrosine phosphatase receptor type sigma (PTPσ) to guide the directionality of migration and initiate the movement of their cell body.
The growth cone responds to chondroitin sulfate (CS) through PTPσ and collapses, resulting in inhibition of neuronal migration. In the presence of CS, the growth cones can revert to their extended morphology when they interact with heparan sulfate (HS), thus re-enabling neuronal migration.
“To investigate whether the effect of HS in reversing the inhibitory effect of CS can promote neuronal migration in the injured brain, it was necessary to apply HS-containing biomaterial to the CS-rich injured brain,” Sawamoto said.
Next, they employed HS-containing gelatin-fiber nonwoven fabric, a biomaterial that provides structural scaffolds for cells such as migrating neurons. They showed that the applied HS-containing fibers promoted extension of growth cones and neuronal migration in the injured brain.
Furthermore, implantation of the HS-enriched gelatin fabric promoted the regeneration of mature neurons and restored neurological functions.
These results suggest that elucidating the molecular mechanisms of growth cone-mediated interaction with the local extracellular environment may enable the development of new regeneration technologies based on the promotion of neuronal migration.
Recent studies by other groups have shown that aging alters the physical properties of brain extracellular matrix, including CSPG.
“Given that the growth cone of migrating neurons serves as a primer for neuronal migration under inhibitory extracellular conditions, it is necessary to further investigate whether the growth cone-mediated treatment to recruit new neurons from the endogenous source to the damaged sites is also applicable to aged brains,” Nakajima said.
More information: Chikako Nakajima et al, Identification of the growth cone as a probe and driver of neuronal migration in the injured brain, Nature Communications (2024). DOI: 10.1038/s41467-024-45825-8
Provided by Nagoya City University

Leave a Reply