Researchers uncover lung adenocarcinoma’s early roots, tracing the cancer back to a specific cell type. These insights offer new hope for detection and prevention.
Produced by
Nature Research Custom MediaMD Anderson
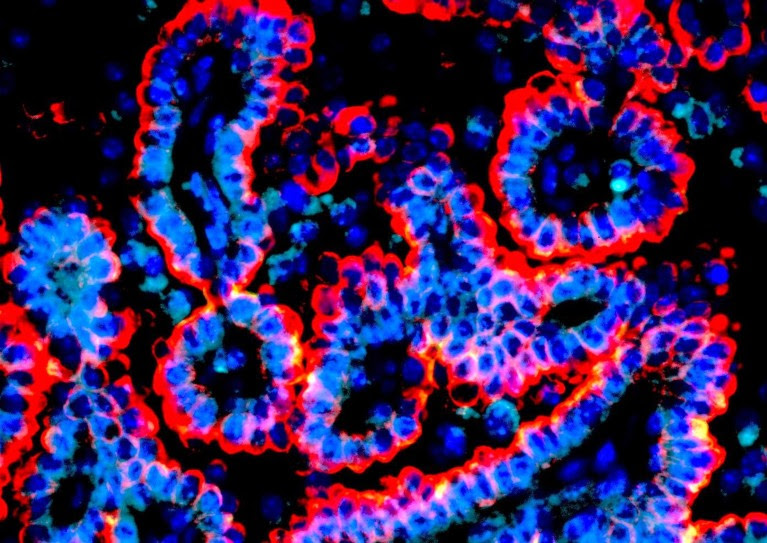
Markers of alveolar intermediate cells, termed KRT8+ alveolar cells (KACs), identified by Kadara and colleagues within a lung tumour. KRT8 in shown in red, DAPI in blue and LAMP3, a marker of AT2 cells, in teal.Credit: Kadara laboratory.
The most common type of lung cancer is a major challenge for clinicians to treat. Lung adenocarcinoma generally forms at the periphery of the lung, making it difficult to detect, and even if caught early, these tumours have a notable tendency to recur. As many as 30–50% of patients may see the disease return after surgery1.
A recent study from researchers led by Humam Kadara, a molecular pathologist at The University of Texas MD Anderson Cancer Center, has revealed important insights into the initiation of lung adenocarcinoma that could inform more effective early treatment. “I believe we’ve hit not only the cell of origin for lung adenocarcinoma, but also the pathway of origin,” says Kadara.
This cancer originates in the epithelial cells that line the lung’s alveoli, the tiny sacs responsible for gas exchange between the bloodstream and inhaled air. Most lung adenocarcinoma studies to date have focused on indiscriminately prepared samples of lung tissue, in which epithelial cells are a minority — Kadara estimates roughly 5-10%, relative to a far larger number of vascular, immune and other cell types.
To rectify this, Kadara teamed up with MD Anderson colleague Linghua Wang, a specialist in cancer genomics. They performed a series of experiments where they extensively profiled gene expression within hundreds of thousands of purified epithelial cells from cancerous and healthy lung tissue samples. Their analysis revealed striking commonalities among tumour cells with mutations in the gene encoding the KRAS protein, a well-known driver of cancer transformation and progression. Further analysis of these tissue samples indicated that these KRAS-mutated cells appear to originate from a specific subpopulation of ‘normal’ alveolar cells with high KRT8 expression, which the group named KRT8+ alveolar intermediate cells, or KACs. This was unexpected, according to Kadara. “These KACs were never seen in lung cancer patients because they were thought to be very transitional,” he says, noting that KACs normally repair lung tissue immediately after injury.
Triggering cancer precursors
To better understand the role of these cells, Kadara’s team performed experiments examining KRAS-mutated lung tumours that arose in mice following exposure to tobacco-derived carcinogens. Once again, they observed the emergence of a sizable population of KACs as a prelude to tumourigenesis, and uncovered evidence that these cells act as direct precursors of malignant cells. This suggests a model in which lung damage from smoking or other carcinogens triggers an abnormal healing response that gets further derailed by KRAS mutation. “KRAS here is an important initiating event — this is like a spark for them to start proliferating,” says Kadara.
There are multiple cancer drugs that specifically target mutated KRAS, but these mostly deliver only limited clinical benefit. If the proposed model proves accurate, Kadara sees exciting opportunities to more effectively apply these drugs at the earliest stages of disease — perhaps even while the injured cells are still in a non-malignant, precancerous state. His team now looks to gain more insight into these early molecular events, and other ways in which the mechanisms underlying lung injury response may contribute to tumourigenesis. “The pathologist Rudolf Virchow once proposed that cancer is caused by irritation and inflammation of the tissues,” says Kadara. “I think there’s a lot of intersection between tissue repair and cancer development.”
References
Kratz, JR, et al. Sci Rep, 11: 23690 (2021)
Google Scholar

Leave a Reply