by Will Doss, Northwestern University
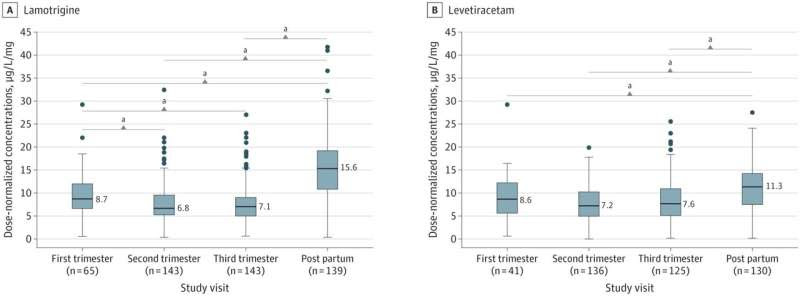
Lamotrigine and Levetiracetam Dose-Normalized Concentrations During Pregnancy and the Postpartum Period Boxplots showing lower dose-normalized concentrations during pregnancy and the postpartum period for lamotrigine (A) and levetiracetam (B). The midline of the box plots indicates the median, and the box indicates the 25th and 75th percentiles. Whiskers represent 1.5 times the IQR. Circles represent outliers. The postpartum period and the first trimester were used as comparators. Credit: JAMA Neurology (2022). DOI: 10.1001/jamaneurol.2021.5487
Women taking anti-seizure medications during pregnancy experienced significant decreases in serum concentrations for such drugs during their pregnancy, according to a study published in JAMA Neurology.
The findings suggest higher doses of those anti-seizure medications may be necessary to maintain seizure control while pregnant, according to Elizabeth Gerard, MD, associate professor of Neurology in the Division of Epilepsy & Neurophysiology and a co-author of the study.
“This study underscores the importance of therapeutic drug monitoring, the practice of checking anti-seizure medications before and during pregnancy and making dose adjustments accordingly,” Gerard said.
Research over the past two decades—mostly with the medication lamotrigine—has shown that some anti-seizure medications can be metabolized faster during pregnancy, according to Gerard. Prior to these studies, pregnant patients were monitored using “clinical features monitoring,” in which providers keep patients on pre-pregnancy doses of anti-seizure medications or treatments until signs or symptoms indicated an adjustment was needed—in this case, providers would wait until a seizure occurred to adjust medications in pregnancy.
“This approach allows for more seizures in pregnancy and puts mother and infant at risk, particularly if the seizures are convulsive,” Gerard said.
In the current cohort study, investigators examined drug concentration of several other anti-seizure medications in pregnant patients with epilepsy and in non-pregnant women with epilepsy. The study enrolled 430 total patients and drug concentration was measured via blood draw during seven visits over 18 months.
Dose-normalized concentrations—concentrations relative to a patient’s non-pregnant baseline—significantly decreased over the course of pregnancy for lamotrigine, levetiracetam, lacosamide and zonisamide. In contrast to other medications, concentrations of unbound carbamazepine and a carbamazepine metabolite did not change significantly during pregnancy.
These results suggest that increased drug metabolism during pregnancy is more widespread among anti-seizure medications than previously thought, according to Gerard, and active drug monitoring should be standard for these patients. Providers should establish a therapeutic baseline for a patient prior to pregnancy and check levels throughout the pregnancy, typically every month, at the same time of day.
Patients taking carbamazepine may not need as frequent monitoring, but those make up a minority of pregnant patients who are prescribed anti-seizure medication, Gerard said.

Leave a Reply