A new wave of research is unpicking the relationship between cancer and neurons — and looking for ways to stop the crosstalk.

McKenzie Prillaman
A 3D model system shows how nerve cells (magenta) interact with cancer cells (green).Credit: Jennifer Su, Peter Wang, Nicole Lester, William L. Hwang
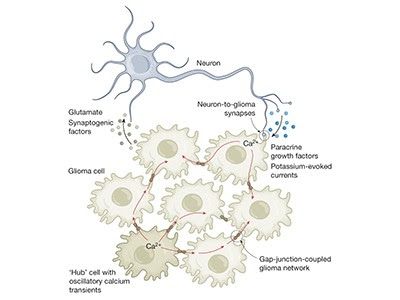
Lightning bolts of lime green flashed chaotically across the computer screen, a sight that stunned cancer neuroscientist Humsa Venkatesh. It was late 2017, and she was watching a storm of electrical activity in cells from a human brain tumour called a glioma.
Venkatesh was expecting a little background chatter between the cancerous brain cells, just as there is between healthy ones. But the conversations were continuous, and rapid-fire. “I could see these tumour cells just lighting up,” says Venkatesh, who was then a postdoctoral researcher at Stanford University School of Medicine in Stanford, California. “They were so clearly electrically active.”
She immediately began to think about the implications. Scientists just hadn’t considered that cancer cells — even those in the brain — could communicate with each other to this extent. Perhaps the tumour’s constant electrical communication was helping it to survive, or even to grow. “This is cancer that we’re working on — not neurons, not any other cell type.” To see the cells fizz with so much activity was “truly mind blowing,” says Venkatesh, who is now at Harvard Medical School in Boston, Massachusetts.
Venkatesh’s work formed part of a 2019 paper in Nature1, which was published alongside another article2 that came to the same conclusion: gliomas are electrically active. The tumours can even wire themselves into neural circuits and receive stimulation directly from neurons, which helps them to grow.

Cancer cells have ‘unsettling’ ability to hijack the brain’s nerves
The findings have been pivotal in the emerging field of cancer neuroscience, in which researchers are parsing the many ways in which cancer — even outside the brain — co-opts the nervous system for its own benefit. In much the same way as tumours recruit blood vessels to feed themselves and grow, cancer relies on the nervous system for everything from initiation to spread.
The interaction between oncology and neuroscience is just beginning to unravel in this once-overlooked part of the tumour’s environment. Scientists are starting to understand which neurons and signals are involved, but new-found interactions with the immune system are making the story even more complicated. As researchers dig deeper into the relationship between cancer and the nervous system, therapies that target the connections are emerging. Some of these treatments use existing drugs to improve outcomes in people with cancer.
“Where we’re headed with this is helping patients,” says cancer biologist Erica Sloan at Monash University in Melbourne, Australia. “Yes, there’s the intellectual delight of understanding what goes on at the biology level. But the key goal is, ‘How do we translate this?’”
Invasion and persuasion
Scientists first spotted liaisons between cancer cells and neurons almost 200 years ago. In the mid-nineteenth century, French anatomist and pathologist Jean Cruveilhier described a case in which breast cancer had invaded the cranial nerve responsible for facial movement and sensations.
This was the first account of perineural invasion, in which cancer cells weave in and around nerves — and then spread. The phenomenon is a sign of an aggressive tumour and foreshadows poor health outcomes.
For a long time, scientists and health professionals thought that nerves served passively as a highway to transport cancer and its associated pain. Many viewed the nervous system as “the victim — the structure that gets destroyed by or damaged by the cancer”, says neuro-oncologist Michelle Monje at Stanford University School of Medicine, who was Venkatesh’s adviser.
But in the late 1990s, urological pathologist Gustavo Ayala, now at the University of Texas Health Science Center at Houston, started investigating the interaction a little more closely. He placed mouse nerves in dishes speckled with human prostate cancer cells. Within 24 hours, the nerves began growing little branches called neurites, which reached out towards the diseased cells. Once they made contact, the cancer travelled along the nerves until it reached the neuronal cell bodies3.
Nerves weren’t just bystanders: they actively sought a connection with cancer. “I thought it was real, and I decided to make it my career,” says Ayala. He soon became known as ‘the nerve guy’. “People didn’t quite make fun, but they didn’t share my interest in the field,” he says.
The neuroscience of cancer
In 2008, Ayala reported another strange phenomenon. Prostate-cancer tumours taken from people who underwent surgery contained more nerve fibres, known as axons, than did samples from healthy prostates4.
Not everyone found this result odd, however. Some scientists were starting to view tumours as being organs themselves, because they contain multiple cell types, a scaffolding structure, blood vessels and other elements that distinguish them from being clumps of cancer cells.
But “there was a piece missing in the landscape — it was nerves”, says Claire Magnon, a cancer biologist at the French National Institute of Health and Medical Research in Paris.
That hunch led to a groundbreaking paper in 2013. She and her colleagues documented nerve fibres sprouting in and around prostate tumours in mice5. Moreover, severing the connections to the nervous system brought the disease to a standstill. In a few years, an avalanche of research demonstrated the same thing happening in cancers elsewhere, including in the stomach, pancreas and skin. Some of the severed nerves carry cancer-associated pain, and researchers already knew that blocking those paths in people with pancreatic cancer could bring some relief.
“The stars were sort of aligned,” says neuroscientist Brian Davis at the University of Pittsburgh in Pennsylvania. The converging results showed “that this component of the tumour microenvironment, that had basically been ignored, was playing some role”.
Hitting a nerve
But where these cancer-infiltrating nerves came from baffled researchers. Work conducted in the following years suggested that cells in the tumour can turn into neurons, or at least acquire neuron-like features. And in 2019, Magnon and her colleagues reported another origin6. They saw cells called neural progenitors travelling through the blood to prostate tumours in mice, where they settled and fledged into neurons. Somehow, cancers were influencing the brain region that contains these cells — an area called the subventricular zone. In mice, these cells are known to help heal certain brain conditions, such as strokes. Some evidence suggests that the same region produces neurons in adult humans, although the idea is controversial.
The following year, another team discovered that cancer can force neurons to change their identities. In a study of oral cancer in mice, researchers found that a group of nerves that relay sensations to the brain, called sensory neurons, acquired features of a different type of neuron that is usually rare in the oral cavity: sympathetic neurons, which are responsible for the ‘fight or flight’ response7.
Coloured scanning electron micrograph of ependymoma cell and a natural killer cell
An immune cell (left) next to a cell from a nervous-system cancer called an ependymoma.Credit: Eye of Science/Science Photo Library
“Now they’re wearing two hats,” says cancer neuroscientist Moran Amit at the University of Texas MD Anderson Cancer Center in Houston, who co-led the study. The transformation might help tumour growth, because sympathetic nerves have been shown to benefit certain cancers.
But the relationships between nerve types and their effects on tumours are complicated. In the pancreas, for instance, a push and pull exists between two types of nerve that have opposite effects on tumours. Sympathetic nerves participate in a vicious feedforward loop that aids the growth of cancer. They release signals that instruct diseased cells to secrete a protein called nerve growth factor, which draws in more nerve fibres. Their counterparts — parasympathetic nerves, which are responsible for the ‘rest and digest’ response — send chemical messages that thwart disease progression.
But in stomach cancer, parasympathetic signals act in the opposite way, encouraging the tumour to grow. And in prostate cancer, both types of nerve aid tumours, with sympathetic nerves helping during the early stages of cancer development and parasympathetic nerves boosting later-stage spread.
“Every cancer is a little bit different in how it interacts with the nervous system,” says gastroenterologist Timothy Wang at Columbia University in New York City. This means that treatment targets must be specific to the type of cancer and how the cancer connects with or uses the nervous system.
Neurons can have direct effects on cancers, or they can act indirectly, by damping down the immune system so that it can’t fight tumours as effectively. A 2022 discovery hints at one such mechanism: a chemical called calcitonin gene-related peptide (CGRP), which is released by sensory nerves, can quell the activity of certain immune cells, making them ill-prepared to ward off cancer8.
Neurons can suppress immune-cell activity to keep themselves safe, because too much inflammation can harm them. So, not only do nerves provide a route and scaffolding for cancer’s spread, says cancer neuroscientist Jami Saloman at the University of Pittsburgh, but they also seem to provide a safe harbour.
A tumour can “tuck itself into the nerves”, Davis says, where it is protected from both the immune system and medication because drugs have a hard time entering nerves. “The cancer cells can hang out while they’re waiting for the storm of biologics and chemotherapy to pass,” he notes. “And then they can re-emerge.”
Central takeover
Some of the most aggressive cancers affect the brain. As Venkatesh and others found, cancer cells even form direct synapses with neurons, the signals of which help them to grow.
A paper published alongside the two 2019 brain-cancer papers showed that breast-cancer metastases in the brain could also form synapse-like connections9. And previous research has linked brain metastases with cognitive impairment.
There are yet more ways in which brain cancers seem to act like brain cells. Last November, Monje’s laboratory reported that gliomas strengthen their neuronal input using a classic brain-signalling method10. When exposed to a protein that helps neurons to grow, called brain-derived neurotrophic factor, glioma cells respond by spawning more receptors that can receive signals from neurons.
“It’s exactly the same mechanism that healthy neurons use in learning and memory,” Monje says. “Cancer doesn’t really invent anything new — it just hijacks processes that already exist.”
How thought itself can drive tumour growth
Furthermore, just like in networks of neurons, some glioma cells can generate their own rhythmic waves of electrical activity11. “They are simply like little beating hearts,” says Frank Winkler, a neuro-oncologist at the German Cancer Research Center in Heidelberg, whose lab conducted the work.
Those electrical surges radiate throughout the cancer cells using a network of thin, stringy bridges called tumour microtubes, which Winkler’s group started studying several years ago. The activity choreographs cancer-cell proliferation and survival — just as pacemaker neurons orchestrate activity during the formation of neural circuits. “Yet again, cancer is hijacking an important neural mechanism of neurodevelopment,” Winkler says.
Brain cancers can even have effects on whole networks. A study last May found that gliomas can reshape entire functional circuits in the brain12. People with tumours that infiltrated speech-production areas were asked to name items described in audio or shown in pictures. Electrodes on the surface of their brains showed that the language task didn’t just stimulate those key language regions — the entire tumour-infiltrated area, including regions not usually involved in speech production, spiked in activity as well. The more functionally connected the tumour was to the rest of the brain, the worse people did on the task, and the less time they were expected to live.

“The tumour had remodelled the functional language circuitry to feed itself,” says Monje, who co-authored the work. She remembers her horror when she looked at the results. “I get goosebumps when I think about the first time I saw that data.”
Bench to bedside and beyond
These initial discoveries are already pointing to potential cancer treatments. They also hint at why existing options often bring brain-draining side effects. Many people undergoing chemotherapy experience cognitive decline, or ‘chemo brain’, and degeneration of nerve fibres elsewhere in the body, says Venkatesh.
Despite being an effective way to attack cancer, if chemotherapy destroys neurons elsewhere in the body, “that’s quite obviously not good for the patient”, she adds.
One tactic is to target specific prongs of the nervous system. And existing therapies might be able to help. “We have the drugs to target almost every branch of the nervous system,” Amit says. “Most of those drugs have a very established safety profile.”
Beta blockers, for instance, can disrupt signals from sympathetic nerves that drive cancer progression in the breast, pancreas, prostate and elsewhere. These drugs have been used to treat heart problems such as high blood pressure, and sometimes also anxiety, since the 1960s.
Sloan has wanted to repurpose the drugs for the past decade, but at first she faced resistance. People often remarked, “If beta blockers were going to do anything to cancer, we would know that already,” she recalls.
To explore the connection, she led a phase II clinical trial, published in 2020, testing the beta blocker propranolol in people with breast cancer. Taking the medication for just one week reduced signs of the cancer’s potential to metastasize13. Another phase II trial, inspired by observational studies that have linked beta-blocker use to better health outcomes, demonstrated that it was safe to combine chemotherapy and propranolol in people being treated for breast cancer14. And last year, Sloan found that the drug enhances a common chemotherapy treatment15.
Other researchers are repurposing drugs that interrupt neuronal communication, including medications developed for seizures and migraine. At least one clinical trial is aiming to block the synapses formed between neurons and cancer cells in gliomas using an anti-seizure drug, which calms hyperexcitable cells.
Another trial in the planning stages will look at whether people receiving immunotherapy for skin or head-and-neck cancer would also benefit from taking a migraine medication. It’s thought that migraines can be triggered by high levels of CGRP, the molecule that can blunt the activity of some immune cells in cancer. So the medication, which blocks CGRP receptors, could counteract CGRP and allow immune cells to help fight cancer again.
Venkatesh imagines that a cocktail of drugs with complementary effects will probably be needed to control the disease. “There is really no silver bullet,” she says.
The field is only just beginning to unravel this insidious relationship, and questions abound. “I think I would need 50 lives to go after all of them,” Winkler says.
Nature 626, 22-24 (2024)
doi: https://doi.org/10.1038/d41586-024-00240-3
References
Venkatesh, H. S. et al. Nature 573, 539–545 (2019).
Article
PubMed
Google Scholar
Venkataramani, V. et al. Nature 573, 532–538 (2019).
Article
PubMed
Google Scholar
Ayala, G. E. et al. Prostate 49, 213–223 (2001).
Article
PubMed
Google Scholar
Ayala, G. E. et al. Clin. Cancer Res. 14, 7593–7603 (2008).
Article
PubMed
Google Scholar
Magnon, C. et al. Science 341, 1236361 (2013).
Article
PubMed
Google Scholar
Mauffrey, P. et al. Nature 569, 672–678 (2019).
Article
PubMed
Google Scholar
Amit, M. et al. Nature 578, 449–454 (2020).
Article
PubMed
Google Scholar
Balood, M. et al. Nature 611, 405–412 (2022).
Article
PubMed
Google Scholar
Zeng, Q. et al. Nature 573, 526–531 (2019).
Article
PubMed
Google Scholar
Taylor, K. R. et al. Nature 623, 366–374 (2023).
Article
PubMed
Google Scholar
Hausmann, D. et al. Nature 613, 179–186 (2023).
Article
PubMed
Google Scholar
Krishna, S. et al. Nature 617, 599–607 (2023).
Article
PubMed
Google Scholar
Hiller, J. G. et al. Clin. Cancer Res. 26, 1803–1811 (2020).
Article
PubMed
Google Scholar
Hopson, M. B. et al. Breast Cancer Res. Treat. 188, 427–432 (2021).
Article
PubMed
Google Scholar
Chang, A. et al. Sci. Transl. Med. 15, eadf1147 (2023).
Article
PubMed
Google Scholar

Leave a Reply