JULY 12, 2024
by Weizmann Institute of Science
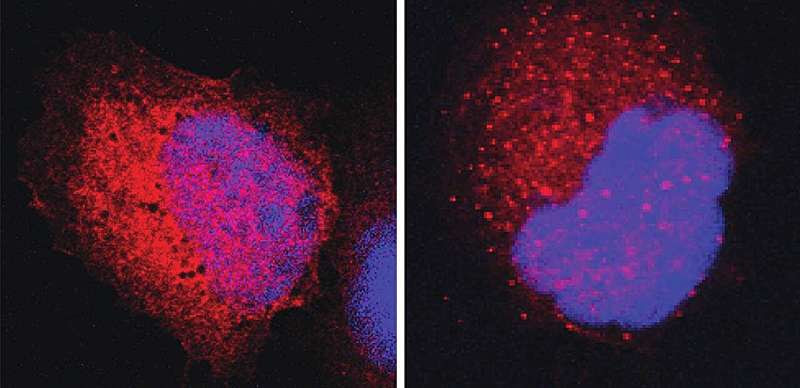
Cells with cancer-causing p53 proteins (red); the genetic material in the cell nuclei is in blue. Left: In the presence of a regular DNAJA2 chaperone, the structure of the p53 proteins is stabilized, so they do not bind with one another and therefore remain scattered throughout the cell. Right: In contrast, in the presence of a DNAJA2 variant without a “hairpin,” the cancer-causing p53 proteins form clusters (red dots) that the cell can recognize and destroy. Credit: Weizmann Institute of Science
“Who will watch the watchers?” asked Roman poet Juvenal way back in the first century C.E. Nature has been addressing that very question for much, much longer. The human body contains proteins that are designed to protect us from cancerous growths.
Like most proteins, to do their job properly, these “guardians” have to fold into a specific three-dimensional structure—and they often need a helping hand to do so. Guarding these guardians, therefore, are chaperone proteins—molecules that ensure that proteins are folded properly so they can function as they are supposed to.
On occasion, genetic mutations in guardian proteins can turn them from inhibitors into promoters of cancer. Unable to discern the change, the chaperones that guard them unfortunately provide them with the same assistance that they do for regular proteins.
In a new study, Dr. Rina Rosenzweig and her research team at the Weizmann Institute of Science have uncovered a mechanism by which chaperones protect a protein with a cancerous mutation. Their findings, published in Molecular Cell, could pave the way for the development of new, targeted cancer treatments.
One of the most common families of chaperones is the J-domain protein (JDP) family. Over the past few decades, researchers have discovered that it has around 50 different representatives in the human body.
Their functions include identifying proteins that have not folded properly or in which the structure has come apart, and sending them to be refolded with the help of other chaperones. Among their other tasks, members of the JDP family assist in the folding of the p53 protein, which is known as the “guardian of the genome.”
In its regular form, this genome guardian inhibits cancerous growths, but tiny genetic changes that replace one of its constituent amino acids can cause it to promote cancer instead. Previous studies have shown that the guardians of the genome guardian—that is, the chaperones—provide protection not only to the properly functioning p53 but also to its cancerous version.
Chaperones stabilize the cancerous proteins’ unstable structure and prevent them from adhering to each other and forming disordered aggregates that cells would normally spot and dismantle, were it not for that very assistance.
It seems, therefore, that chaperones could be a good target for the development of new cancer therapies. However, because they provide assistance to a large range of proteins in the cell, harming the chaperones could lead to serious secondary damage.
Researchers from Rosenzweig’s lab in the Chemical and Structural Biology Department led by Dr. Guy Zoltsman, with the participation of Miriam Kuchersky and Dr. Ofrah Faust, postulated that studying members of the JDP family that provide assistance to cancerous versions of the p53 protein could uncover a new target for focused cancer treatment.
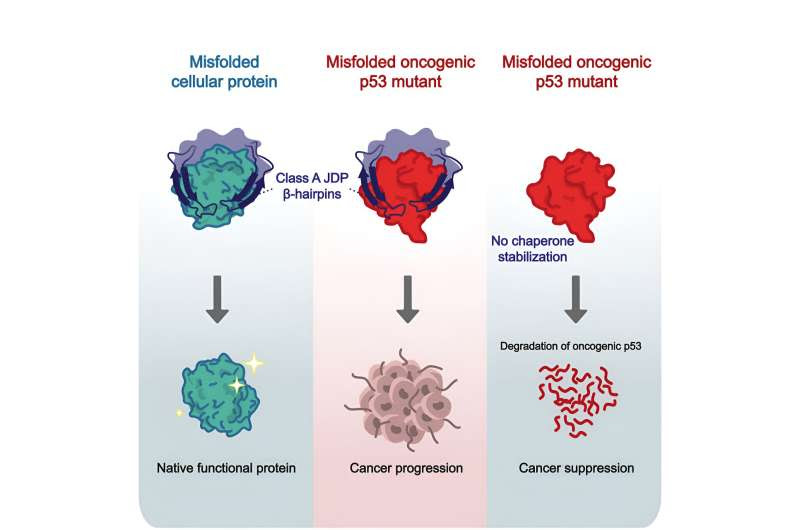
Credit: Molecular Cell (2024). DOI: 10.1016/j.molcel.2024.02.018
The first step was to identify exactly which members of the family assist the mutated, cancerous p53. To do so, the researchers examined four groups of proteins from the JDP family that had been shown to have an impact on the progression of cancer. These experiments revealed that only chaperone proteins from Class A, particularly one called DNAJA2, helped the mutated p53.
These findings were also verified in cancerous cells, thanks to a joint research project with the laboratory of Prof. Bernd Bukau from the German Cancer Research Center (DKFZ) in Heidelberg. But how does DNAJA2 identify and protect the cancerous p53 protein?
Using cutting-edge nuclear magnetic resonance (NMR) technology at Weizmann’s Clore Institute for High-Field Magnetic Resonance Imaging and Spectroscopy, the researchers managed to uncover DNAJA2’s mechanism of action.
Most proteins in a cell are created as molecular chains that fold themselves into a three-dimensional structure in which the water-loving components are located on the outside of the protein’s structure, facing the cell’s liquid environment, while the water-repellent components are located in the protein’s inner part.
Under normal circumstances, by identifying water-repellent areas that have become exposed on the protein’s surface, chaperones can recognize a protein that has not folded properly or has lost its normal three-dimensional shape.
“Unlike the rest of the chaperones, DNAJA2 binds with p53 when it is almost fully folded,” Rosenzweig explains. “It turns out that it is capable of identifying proteins in which the three-dimensional structure has only just started to come apart—long before whole internal areas are exposed.”
Using NMR, the researchers were able to analyze the interaction between DNAJA2 and the p53 protein down to the level of individual atoms. This revealed that hairpin-like sections of the chaperone—which are therefore known as β-hairpins (beta hairpins)—attach to areas that look like accordions (known as β-sheets) in the core of the target protein.
The structure of the β-sheets is strengthened by hydrogen bonds, which remain stable throughout the functional life of the protein. However, when these bonds come loose—as happens in the cancerous version of the genome guardian—they increase the risk that the protein will stick to other proteins.
This is where the hairpins come into play: They bond with these loosened areas, stabilize them and give them the time to rebuild the hydrogen bonds. This protection afforded to the cancerous proteins prevents the cell from identifying them and breaking them down.
When the researchers deleted the recipe for the hairpin from the chaperones’ genetic code, they discovered that the chaperones remained functional and the deletion damaged only their binding to proteins that are especially rich in accordion sheets, such as p53.
“Since the activity of the ‘hairpin’ is so focused, it seems that we should be able to develop treatments for cancer by targeting specific regions in specific chaperones, without causing significant harm to the functioning of the body’s cells,” says Rosenzweig. “Our study presents the potential target of such treatments, which would reduce DNAJA2’s cancer-supporting activity.”
More information: Guy Zoltsman et al, A unique chaperoning mechanism in class A JDPs recognizes and stabilizes mutant p53, Molecular Cell (2024). DOI: 10.1016/j.molcel.2024.02.018
Journal information: Molecular Cell

Leave a Reply