by Levi Gadye, University of California, San Francisco
Images by Swanson Lab
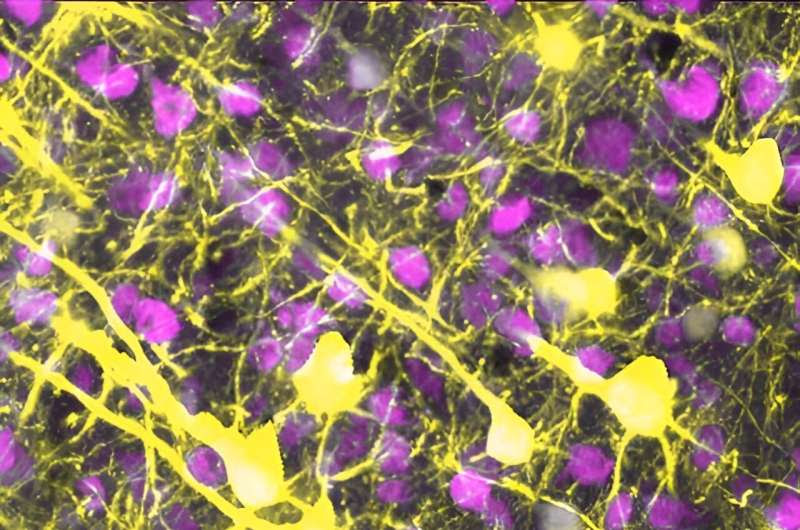
The brain’s neurons (yellow) connect with one another using a vast network of neural wires, called neurites. Credit: Swanson Lab/University of California, San Francisco
Whether reeling from a sudden stroke or buckling under the sustained assault of Alzheimer’s, the brain becomes inflamed, leading to cognitive problems and even death.
Scientists have known for many years that severe inflammation can kill the brain’s neurons. Now, researchers at UC San Francisco have discovered that even subtle inflammation damages the brain.
Instead of killing neurons outright, however, relatively mild inflammation only destroys the arm-like projections, called neurites, that wire neurons together. These connections are vital for everything the brain does, including learning and memory.
The findings, published last month in Cell Reports, describe in detail a new degenerative pathway that scientists can now try to disrupt. This could help stem the damage from common neurological diseases.
“There are several exciting drugs now entering clinical use that interrupt these inflammatory processes, and now we know to look at their effects on neurites,” said Raymond Swanson, MD, senior author on the paper and a professor of neurology with joint appointments at UCSF and the San Francisco Veterans Affairs Medical Center. “Not too far off, this could have a big impact on helping our patients.”
Ray Swanson’s team discovered that inflammation—a consequence of diseases like Alzheimer’s and stroke—destroys these wires but spares the neurons. Credit: Swanson Lab/University of California, San Francisco
Friendly fire from the immune system
Inflammation is the body’s first line of defense when something goes wrong. It rushes blood to an injured area, bathing it with immune cells that release chemicals to kill pathogens.
The strategy works well against bacteria, but it’s brutal on the brain’s delicate neural networks.
Swanson’s team wanted to know how this inflammatory process was damaging the brain. They were particularly interested in molecular aggregates, called cofilactin rods (CARs), that appear after a stroke. CARs form when two proteins, called cofilin and actin, that normally maintain neurites, break loose, forming messy clumps.
CARs are known to form in response to a chemical called superoxide, which immune cells release when the brain is inflamed.
In laboratory animals treated with increasing doses of the inflammatory molecule, S100β, the healthy web of neurites slowly deteriorates, leaving the neurons disconnected. Credit: Swanson Lab/University of California, San Francisco
Connecting the dots from inflammation to dysfunction
To get a closer look at this process, the researchers stimulated inflammation in a part of the mouse brain that controls movement. They expected that neurons would die and the mice would have trouble moving.
The mice did struggle to move, but when the researchers looked at their brain tissue under a microscope, they were surprised to see that only the neurites had withered away, leaving the neurons isolated like stars in the night sky. The loss of these connections was enough to rob the mice of some of their motor coordination.
The scientists then tried reducing the amount of either superoxide or cofilin, and treated the brain with the same inflammatory substance. Under these conditions, fewer CARs formed, and the neurites survived. The mice also retained their coordination.
They had discovered a new pathway: inflammation caused immune cells to release superoxide, pulling cofilin and actin out of neurites and making CARs. Neurites died, and the disconnected brain malfunctioned.
A new target for treating neurodegeneration
Many neurological diseases involve inflammation, including multiple sclerosis, traumatic brain injury, and amyotrophic lateral sclerosis (ALS).
Now that scientists understand it better, they can design therapies to interrupt this inflammatory pathway. Stroke patients, for example, could be treated early on with anti-inflammatory agents to shield neurites from damage and preserve cognition.
“Particularly in the aging brain, inflammation can be harmful,” Swanson said. “By homing in on how neurites are so vulnerable to inflammation, we may be able to finally gain the upper hand against some of the most common neurological diseases.”
More information: Gökhan Uruk et al, Cofilactin rod formation mediates inflammation-induced neurite degeneration, Cell Reports (2024). DOI: 10.1016/j.celrep.2024.113914
Journal information: Cell Reports
Provided by University of California, San Francisco

Leave a Reply