by Nanjing University School of Life Sciences
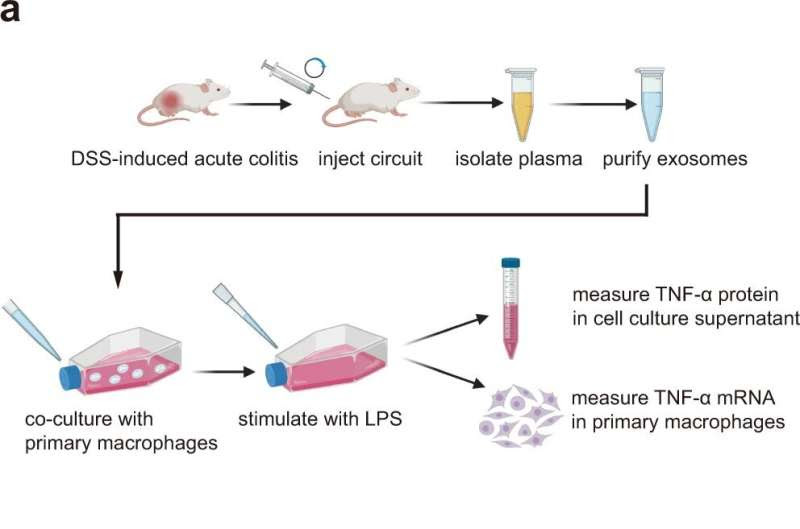
Evaluation of the activity of TNF-α siRNA-encapsulating sEVs in an ex vivo model. a Schematic of the experimental design. Acute UC was induced in male BALB/c mice by replacing their drinking water with a 2.5% DSS solution for 7 days. DSS mice were intravenously injected with 5 mg/kg CMV-scrR or CMV-siRTNF-α circuit every day for a total of 7 times, and then the sEVs were purified from the plasma of each mouse and dissolved in 50 µL PBS. BCA method was employed to quantify total protein content in sEVs, and the isolated sEVs had a total protein concentration of ~0.8 μg/μL. Subsequently, the sEV solution (50 µL) was incubated with 1 × 105 primary macrophages. After stimulating macrophages with 50 ng/mL LPS, the suppression of TNF-α expression by TNF-α siRNA were examined in this ex vivo model. b Quantitative RT-PCR analysis of the relative expression levels of TNF-α mRNA in primary macrophages (n = 6 in each group). Created with BioRender.com. c Determination of the levels of secretory TNF-α protein in cell culture supernatant by ELISA (n = 6 in each group). Values are presented as the mean ± SEM. Significance was determined using one-way ANOVA followed by Dunnett’s multiple comparison. **p < 0.01; ***p < 0.005. Credit: Nature Communications (2022). DOI: 10.1038/s41467-022-33436-0
Despite recent advancements in our knowledge of the pathogenic mechanisms of ulcerative colitis, there is still a considerable unmet medical need for the treatment of the condition.
Because ulcerative colitis is a multifactorial and multistep process, merely blocking a single inflammatory cytokine or immunological target may not be adequate to cure this disease. Combination therapy targeting multiple pathogenic genes and pathways of ulcerative colitis may be required.
Unfortunately, current therapeutic strategies are usually based on independent chemical compounds or monoclonal antibodies, and the full potential of combination therapy has not yet been realized for the treatment of ulcerative colitis.
Due to the high specificity, potency and flexibility of small interfering RNAs (siRNAs) to cotarget multiple genes at a single dosage, siRNA-based therapeutics offer an attractive strategy for combination therapy of ulcerative colitis. However, the development of an appropriate in vivo delivery system for siRNAs remains a major bottleneck of RNAi therapy.
In a new study published in Nature Communications, a joint research group led by Prof. Chen-Yu Zhang, Xi Chen and Qipeng Zhang at Nanjing University developed a synthetic biology strategy that integrates the naturally existing circulating system of small extracellular vesicles with artificial genetic circuits to reprogram the liver of male mice to self-assemble multiple siRNAs into secretory small extracellular vesicles and facilitate in vivo delivery siRNAs through circulating small extracellular vesicles for the combination therapy of mouse models of ulcerative colitis.
Particularly, repeated injection of the multi-targeted genetic circuit designed for simultaneous inhibition of TNF-α, B7-1 and integrin α4 rapidly relieved intestinal inflammation and exerted a synergistic therapeutic effect against ulcerative colitis through suppressing the pro-inflammatory cascade in colonic macrophages, inhibiting the costimulatory signal to T cells and blocking T cell homing to sites of inflammation.
More importantly, they designed an AAV-driven genetic circuit to induce substantial and lasting inhibition of TNF-α, B7-1 and integrin α4 through only a single injection. Overall, this study established a feasible combination therapeutic strategy for ulcerative colitis, which may offer an alternative to conventional biological therapies requiring two or more independent compounds or antibodies.
This study is important for the following reasons:
(1) The multitargeted genetic circuit is formed as naked DNA plasmid or AAV and simply administered through intravenous injection; then, multiple siRNAs are spontaneously and simultaneously produced by the liver and transferred through the circulating system of small extracellular vesicles. This design borrows the body’s own small RNA assembly and transport machineries and reconceptualizes the strategy of combination therapy, thereby addressing the bottleneck problem in combination therapy of ulcerative colitis.
(2) In vivo self-assembled siRNA alleviates the characteristic symptoms of ulcerative colitis to a better extent as TNF-α antibody infliximab. Since monoclonal antibodies suffer from some inherent limitations of antibody drugs (e.g., high costs, serious side effects and generation of anti-antibodies) in vivo self-assembled siRNA holds strong promise of becoming a new option for patients with ulcerative colitis.
(3) This study provides the groundwork for delivery of siRNAs to inflamed sites and immune cells to fine-tune immune responses. Since overactive immune response is the hallmark of many autoimmune diseases, in vivo self-assembled siRNA is expected to accelerate the development of new therapies for other autoimmune diseases (e.g., systemic lupus erythematosus and rheumatoid arthritis).

Leave a Reply