July 26, 2024
by Tokyo Medical and Dental University
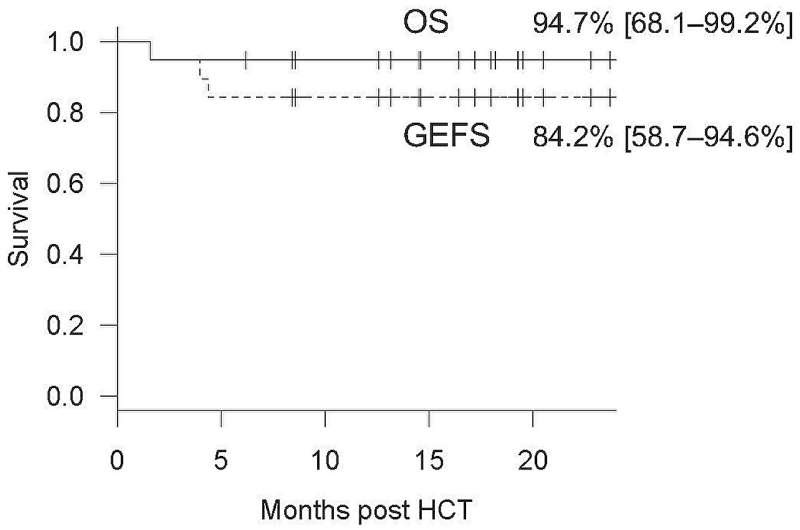
The OS and GEFS of the study cohort. The solid line and the dashed line show OS and GEFS curves, respectively. OS and GEFS at 2 years post-HCT are described with 95% confidence intervals in brackets. Grade III–IV acute GVHD, systemically treated chronic GVHD, second transplantation, and death were defined as failure events for GEFS. OS, overall survival; GEFS, graft-versus-host disease-free, event-free survival. Credit: Department of Child Health and Development, TMDU
Allogeneic hematopoietic cell transplantation (HCT) is a commonly used curative therapy for individuals with inborn errors of immunity (IEI). HCT involves introducing stem cells from a compatible donor with the aim of replacing the affected cells in the recipient’s body. Reduced-toxicity conditioning (RTC) is an approach for reducing drug-related toxicities post HCT in patients with IEIs.
Alemtuzumab is a humanized anti-CD52 monoclonal antibody that strongly suppresses the function of immune cells and is used with RTC regimens to increase the acceptance of transplanted stem cells, thereby reducing graft-versus-host disease (GVHD). Although alemtuzumab is a well-established drug used for RTC in Western countries, the clinical experience of using it on Asian patients with IEIs is limited.
In a study published in the Journal of Clinical Immunology, researchers from Tokyo Medical and Dental University (TMDU) in Japan sought to address this knowledge gap.
The researchers conducted a retrospective analysis of Asian patients who underwent allogeneic HCT alongside RTC with alemtuzumab and shed light on the efficacy and limitations of this drug in an Asian cohort for the first time. They included 19 patients in the analysis who had undergone their first round of HCT conditioning with alemtuzumab in TMDU or collaborative centers.
The donors comprised HLA-half-matched parents, HLA-matched siblings, and unrelated donors. The cohort displayed a diverse range of IEIs, including chronic granulomatous disease, familial hemophagocytic lymphohistiocytosis, leukocyte adhesion deficiency, and X-linked lymphoproliferative syndrome. The diverse range of IEIs helped cement the wide applicability of alemtuzumab. Out of the 19 patients, 18 survived during the follow-up period of a median of 18 months, resulting in an overall survival rate of 94.7% post-HCT.
Highlighting the efficacy demonstrated by alemtuzumab, senior author Prof. Hirokazu Kanegane says, “All surviving patients recovered from the symptoms occurring from the original disease after HCT, including those with active hemophagocytic lymphohistiocytosis, which is life-threatening,”
Except for one patient who passed away, all other patients achieved high levels of neutrophil and platelet engraftment within three months. In other words, the precursors of these cells successfully made their way to the recipient’s bone marrow, where they managed to survive and multiply into healthy immune cells.
However, there were notable variations in donor chimerism—the proportion of grafted donor cells found in the recipient’s body. It was found that six months to a year after HCT, six patients exhibited less than 80% of donor CD3+ T cells in their blood, implying that these immune cells were not as effectively replaced as the other types.
Another important point to mention is that GVHD, which is a typical complication following allogeneic HCT, occurred in acute form in only eight patients and in chronic form in five. Fortunately, the acute GVHD never reached the most severe categories, and only two of those with chronic GVHD had to receive additional immunosuppressive therapy. Among other typical complications of HCT, viral infections were observed in 11 patients, with 6 of them showing a symptomatic infection- a higher frequency when compared with patients who didn’t receive alemtuzumab.
Overall, the researchers demonstrated that RTC with alemtuzumab was safe and effective in patients with IEIs. However, further investigation is necessary to make it even more safe and effective through optimized treatment and management protocols.
“We need to carefully address the development of frequent viral infections and unstable levels of donor T-cell chimerism, emphasizing the importance of monitoring viral status and T-cell-specific chimerism in patients with IEIs treated with HCT using alemtuzumab. Moreover, the optimal dose of alemtuzumab should be further investigated in future prospective studies,” concludes lead author Dr. Satoshi Miyamoto.
More information: Satoshi Miyamoto et al, Allogeneic Hematopoietic cell Transplantation Using Alemtuzumab in Asian Patients with Inborn Errors of Immunity, Journal of Clinical Immunology (2024). DOI: 10.1007/s10875-024-01734-5
Provided by Tokyo Medical and Dental University

Leave a Reply